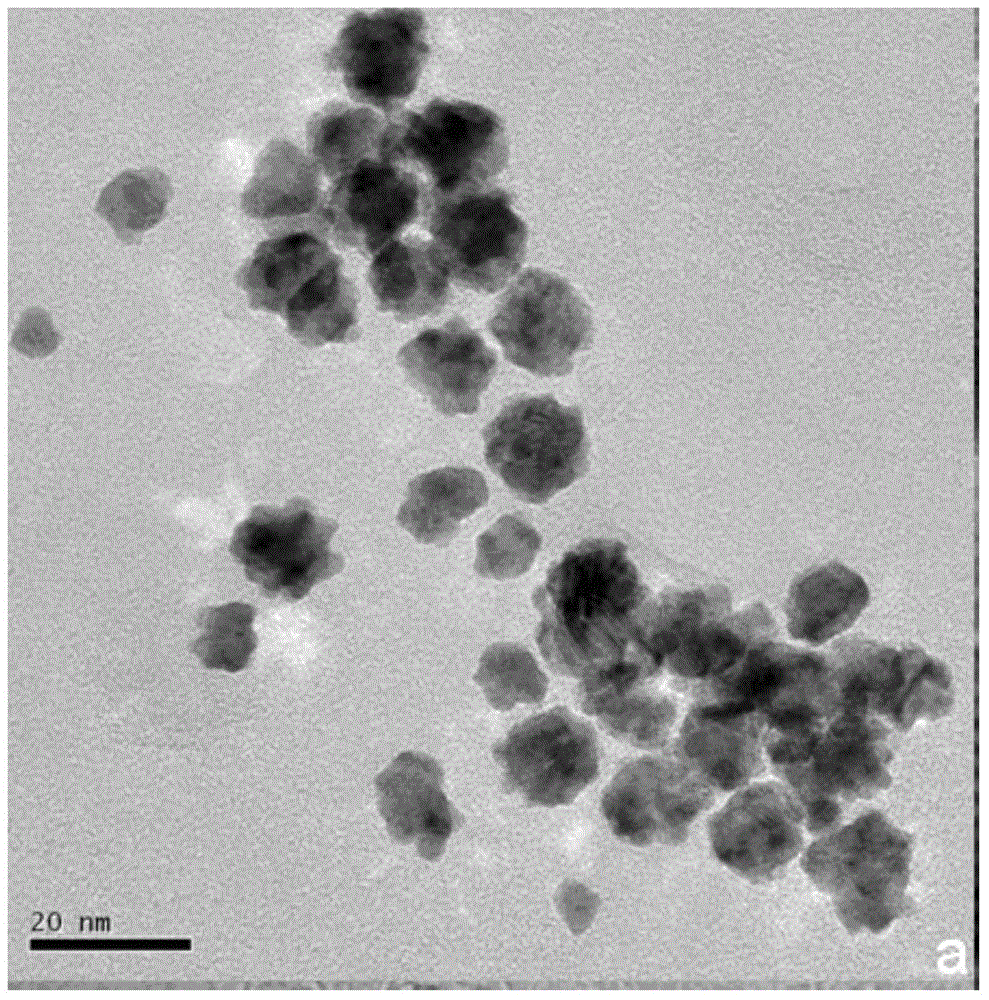
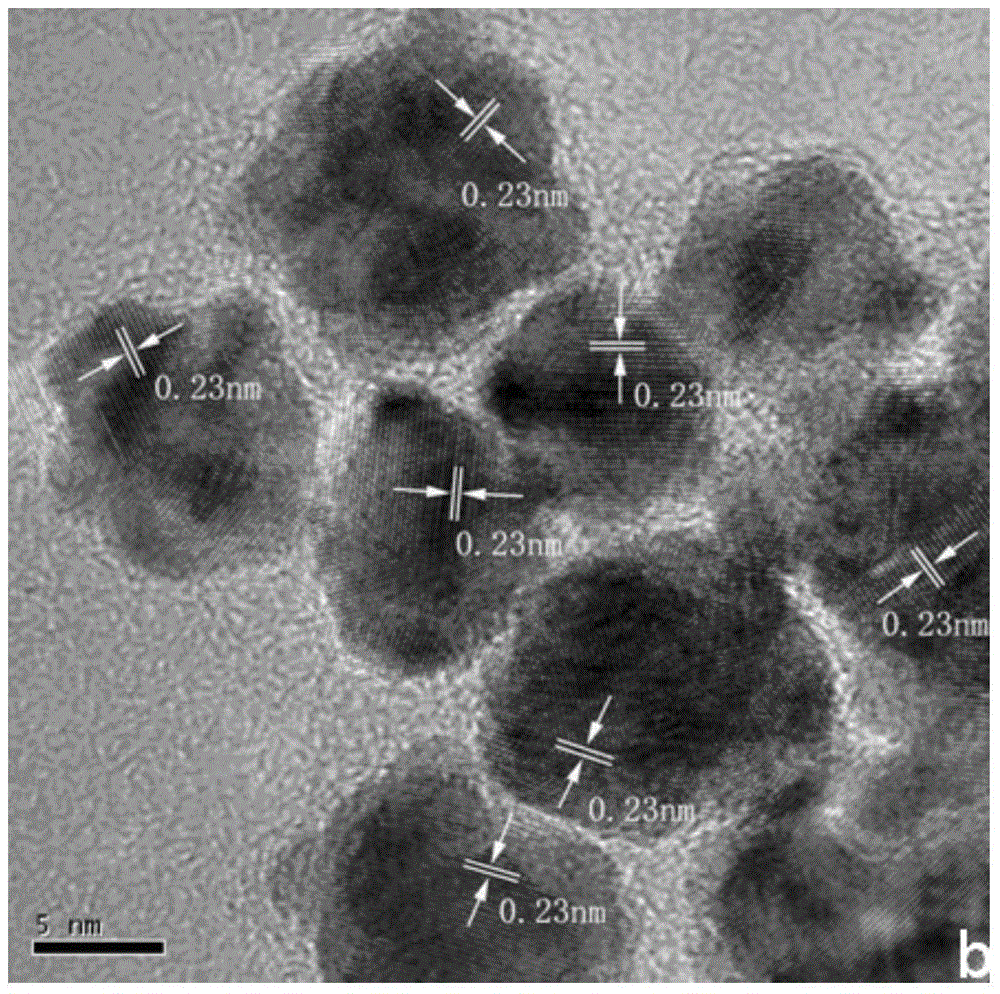
Au@AuPt alloy nanoparticles and preparation method of colloidal dispersion system
A technology of alloy nanoparticles and colloidal dispersion, applied in the field of nanometers, to achieve the effects of easy control of product composition, simple preparation process, and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Prepare HAuCl with a concentration of 0.162mol / L 4 and H 2 PtCl 6 aqueous solution.
[0031] (2) Add 125 μL of HAuCl prepared in step (1) to a 50ml round bottom flask successively 4 aqueous solution, 125 μL H 2 PtCl 6 Aqueous solution, 0.75g sodium diisooctyl sulfonate succinate, 5ml cyclohexane, mixed uniformly to obtain system A.
[0032] (3) Add 500 μL of ascorbic acid aqueous solution with a concentration of 150 g / L to System A under magnetic stirring at room temperature, and the solution gradually turns purple. Continue magnetic stirring for 30 minutes; then add 100 μL of 80% hydrazine hydrate solution, the solution gradually turns brown, react for 30 minutes, and the experiment stops.
[0033] (4) Transfer the reaction system obtained in step (3) to a 50ml centrifuge tube, add 40ml of ethanol, stir well and carry out centrifugal sedimentation, discard the supernatant to get the precipitate, and wash the precipitate repeatedly with absolute ethanol to obt...
Embodiment 2
[0037] (1) Prepare HAuCl with a concentration of 0.162mol / L 4 and H 2 PtCl 6 aqueous solution.
[0038] (2) Add 100 μL of HAuCl prepared in step (1) to a 50ml round bottom flask sequentially 4 aqueous solution, 100 μL H 2 PtCl 6 Aqueous solution, 0.5g sodium dioctyl succinate sulfonate, 5ml cyclohexane, mixed uniformly to obtain system A.
[0039] (3) Add 300 μL of ascorbic acid aqueous solution with a concentration of 100 g / L to System A under magnetic stirring at room temperature, and the solution gradually turns purple. Continue magnetic stirring for 30 minutes; then add 20 μL of 80% hydrazine hydrate solution, the solution gradually turns brown, react for 30 minutes, and the experiment stops.
[0040] (4) Transfer the reaction system obtained in step (3) to a 50ml centrifuge tube, add 40ml of ethanol, stir well and carry out centrifugal sedimentation, discard the supernatant to get the precipitate, and wash the precipitate repeatedly with absolute ethanol to obtain A...
Embodiment 3
[0043] (1) Prepare NaAuCl with a concentration of 0.162mol / L 4 、Na 2 PtCl 6 aqueous solution.
[0044] (2) Add 125 μL of NaAuCl prepared in step (1) to a 50ml round bottom flask in sequence 4 aqueous solution, 125 μL Na 2 PtCl 6 Aqueous solution, 0.75g sodium dioctyl succinate sulfonate, 5ml n-hexane, mixed uniformly to obtain System A.
[0045] (3) Add 500 μL of ascorbic acid aqueous solution with a concentration of 150 g / L to System A under magnetic stirring at room temperature, and the solution gradually turns purple. Continue magnetic stirring for 30 minutes; add 100 μL of 80% hydrazine hydrate solution, the solution gradually turns brown, react for 30 minutes, and the experiment stops.
[0046] (4) Transfer the reaction system obtained in step (3) to a 50ml centrifuge tube, add 40ml of ethanol, stir well and carry out centrifugal sedimentation, discard the supernatant to get the precipitate, and wash the precipitate repeatedly with absolute ethanol to obtain AuAuPt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com