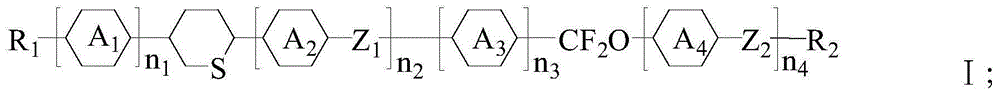Liquid crystal compound containing tetrahydro-thiopyran group and preparation method thereof, and liquid crystal composition
A technology of thiotetrahydropyranyl and liquid crystal compounds, applied in chemical instruments and methods, liquid crystal materials, etc., to achieve the effects of good compatibility, wide nematic phase temperature range, and faster response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Preparation of compound I-a:
[0152] Its synthetic route is:
[0153]
[0154]
[0155] Its concrete preparation steps are as follows:
[0156] 1.1 Preparation of intermediate Ⅰ-a-1:
[0157] Add 9.5g (0.11mol) n-valeraldehyde, 9.5g (0.11mol) methyl acrylate, 16g (0.11mol) trimethylsilyldiethylamine, 1g p-hydroxyanisole and 130ml acetonitrile (solvent) to a 250ml reaction bottle , heated to reflux under the protection of nitrogen, and refluxed for 24 hours; after the reaction, the temperature was lowered, and the solvent was evaporated; then 22ml of glacial acetic acid and 44ml of water were added to the reaction bottle, the temperature was raised again, and the reaction was refluxed for 2h; after the reaction was completed, it was cooled to room temperature, and 100ml of Methyl tert-butyl ether and 100ml water, extract and separate; the aqueous layer is extracted with 50ml×2 methyl tert-butyl ether (that is, each time with 50ml methyl tert-butyl ether, a tot...
Embodiment 2
[0188] Preparation of compound I-b:
[0189] The preparation of compound I-b is carried out using the intermediate I-a-4 prepared in Example 1 as a starting material, and its synthetic route is:
[0190]
[0191] Its concrete preparation steps are as follows:
[0192] 2.1 Preparation of intermediate Ⅰ-a-4:
[0193] Intermediate I-a-4 was prepared according to the preparation method of step 1.1 to step 1.4 in Example 1.
[0194] 2.2 Reactant I-b-1 Preparation of:
[0195] The synthesis was carried out according to the method in the literature Peer.Kirsch et al., Angew.Chem.Int.Ed., 2001.40.1480.
[0196] 2.3 Preparation of compound Ⅰ-b
[0197] Add 29.2g (0.11mol) intermediate I-a-4, 38.9g (0.1mol) reactant I-b-1, 0.3g tetrakistriphenylphosphine palladium, 15g sodium carbonate, 100ml toluene, 100ml Water and 100ml of ethanol were heated to reflux under stirring, and refluxed for 4 hours; after the reaction, the temperature was lowered, and 100ml of water was added f...
Embodiment 3
[0211] Preparation of compound I-c:
[0212] The preparation of compound I-c is carried out using the intermediate I-a-2 prepared in Example 1 as a starting material, and its synthetic route is:
[0213]
[0214] Its concrete preparation steps are as follows:
[0215] 3.1 Preparation of intermediate Ⅰ-a-2:
[0216] Intermediate I-a-2 was prepared according to the preparation method of step 1.1 to step 1.2 in Example 1.
[0217] 3.2 Preparation of intermediate Ⅰ-c-1:
[0218] Add 17.5g (0.1mol) 3-fluorobromobenzene and 250ml tetrahydrofuran into the reaction bottle, stir to dissolve and cool down to -60°C ~ -50°C, and drop 40ml of 2.5M under the condition that the system temperature is not higher than -50°C (0.1mol) of butyllithium; then continue to dropwise add 15.8g (0.1mol) of intermediate Ⅰ-a-2 in tetrahydrofuran (15.8g of intermediate Ⅰ-a-2 dissolved in 50ml of tetrahydrofuran), and stir the reaction for 30min after dropping ;Naturally raise the temperature to -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com