Preparation method of dibenzothiophene derivative
A technology of dibenzothiophene and derivatives, which is applied in the field of preparation of dibenzothiophene derivatives, and achieves the effects of simple preparation method, high yield and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
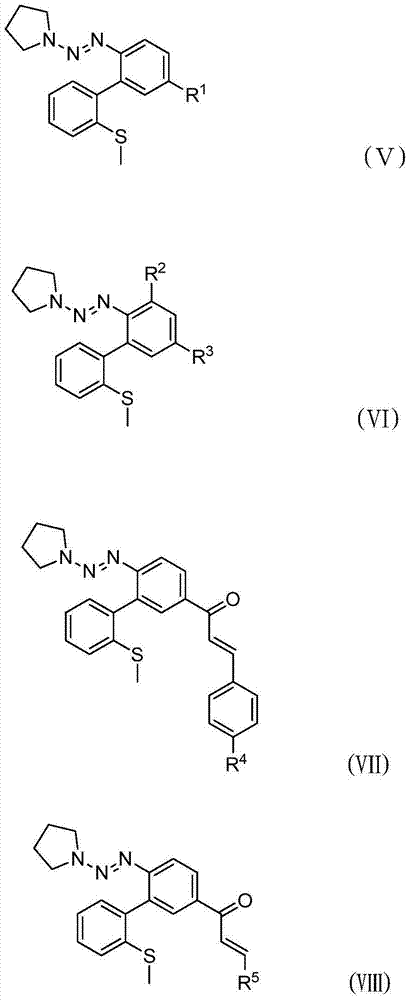
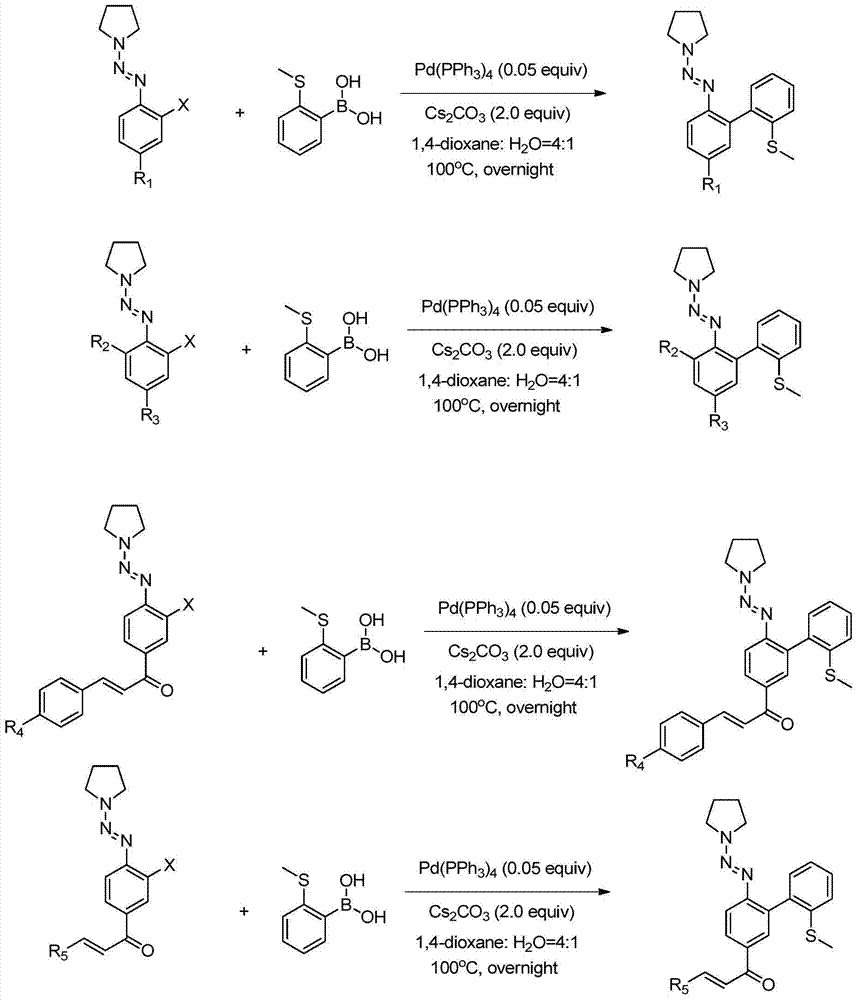
[0031] In a 100mL sealed tube, add 746 mg of 2-halogenated aryl triazene (CAS: 858318-04-4, see the literature for the synthesis method: Organic Letters, 7(13), 2543-2546, 2005), 2-methylsulfide 369 mg of phenylboronic acid, 47 mg of palladium tetrakistriphenylphosphine, and 1.304 g of cesium carbonate, and then 4 mL of 1,4-dioxane and 1 mL of water were added with a syringe. The reaction solution was stirred overnight at 100°C. After the reaction was completed, cool to room temperature, extract with a separatory funnel, concentrate the organic phase and pass through a silica gel column (the volume ratio of petroleum ether to ethyl acetate is 10:1) for purification to obtain 576 mg of product (V-1), with a yield of 78%. , the reaction process is shown in the following formula:
[0032]
[0033] The product prepared in the present embodiment is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0034] 1 H NMR (400MHz, CDCl 3 ): δ8.05 (dd, ...
Embodiment 2
[0037] In a 100mL sealed tube, add 652 mg of 2-halogenated aryltriazene (CAS: 858318-12-4, see the literature for the synthesis method: Organic Letters, 7(13), 2543-2546, 2005), 2-methylsulfide 369 mg of phenylboronic acid, 47 mg of palladium tetrakistriphenylphosphine, and 1.304 g of cesium carbonate, and then 4 mL of 1,4-dioxane and 1 mL of water were added with a syringe. The reaction solution was stirred overnight at 100°C. After the reaction was finished, cool to room temperature, extract with a separatory funnel, concentrate the organic phase and then pass through a silica gel column (the volume ratio of petroleum ether to ethyl acetate is 10:1) for purification to obtain 587 mg of product (V-2), with a yield of 91%. , the reaction process is shown in the following formula:
[0038]
[0039] The product prepared in the present embodiment is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0040] 1H NMR (400MHz, CDCl 3 ):δ7.58-7.60...
Embodiment 3
[0043] In a 100mL sealed tube, add 506 mg of 2-halogenated aryltriazene (CAS: 188966-38-3, see the literature for the synthesis method: Organic Letters, 8(2), 257-260, 2006), 2-methylsulfide 369 mg of phenylboronic acid, 47 mg of palladium tetrakistriphenylphosphine, and 1.304 g of cesium carbonate, and then 4 mL of 1,4-dioxane and 1 mL of water were added with a syringe. The reaction solution was stirred overnight at 100°C. After the reaction was completed, cool to room temperature, extract with a separatory funnel, concentrate the organic phase and pass through a silica gel column (the volume ratio of petroleum ether to ethyl acetate is 10:1) for purification to obtain 476 mg of product (V-3), with a yield of 80%. , the reaction process is shown in the following formula:
[0044]
[0045] The product prepared in the present embodiment is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0046] 1 H NMR (400MHz, CDCl 3 ):δ7.55(d,J=8.0Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com