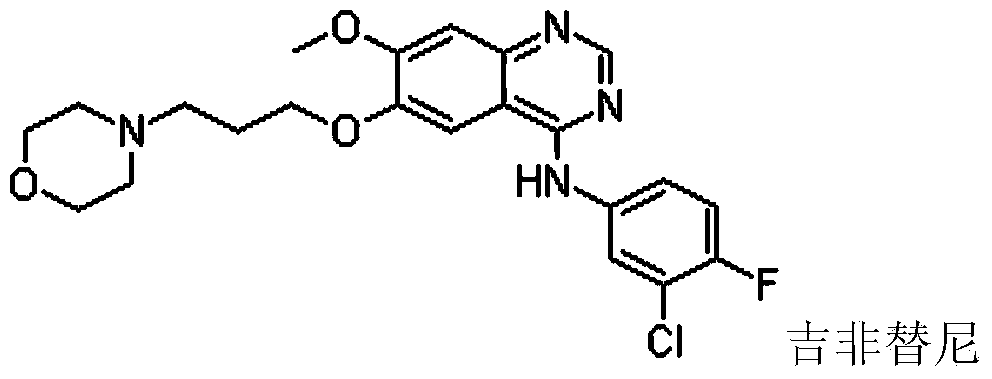
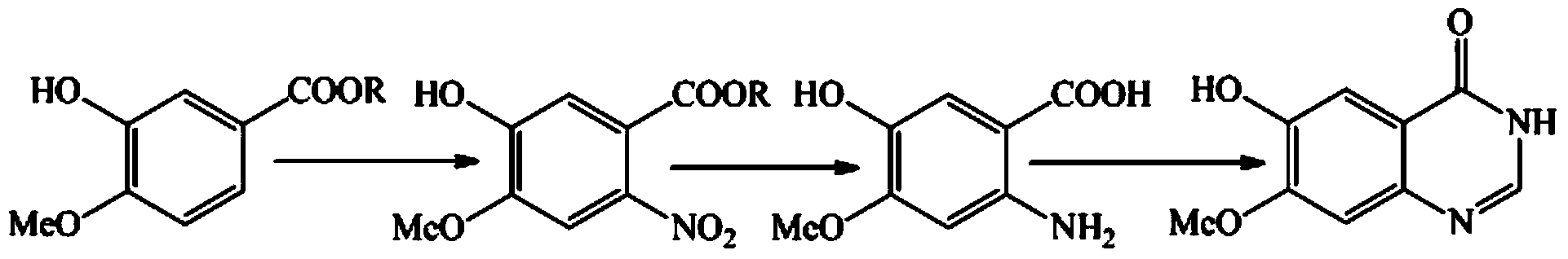
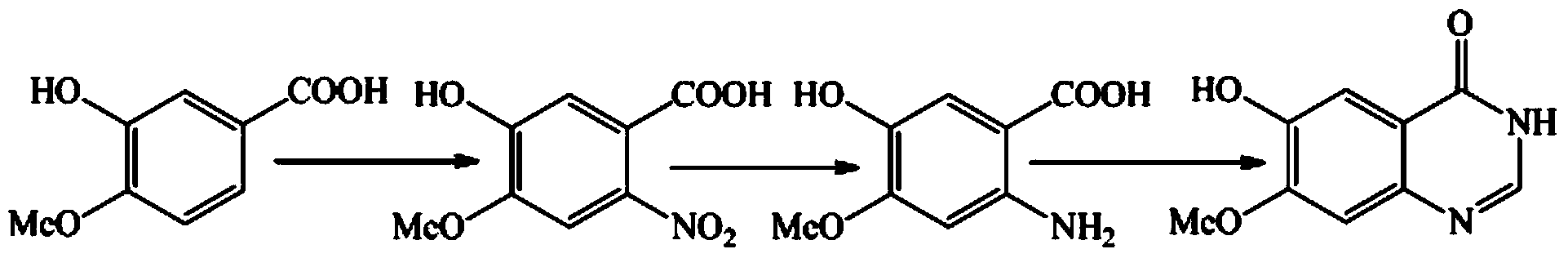
Method for synthesizing gefitinib and intermediate thereof
A synthesis method and technology of gefitinib are applied in the field of synthesis of quinazoline derivatives, can solve problems such as amplification and influence on reaction scale, and achieve the effects of shortening reaction route, improving reaction yield and overcoming dangerous raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 (divided into Examples 1-1 to 1-12 according to different reaction conditions)
[0032] experimental method
[0033] Put compound 5 and formamidine acetate as reactants into a polar aprotic solvent, add L-proline and Cu(I) salt, and heat to the reaction temperature under the protection of nitrogen until the reaction is complete. Ethyl acetate was added and the layers were washed with water. The organic phase was collected, filtered, concentrated under reduced pressure, and the crude product was refined to obtain compound 5.
[0034] Compound 5 was prepared from compound 4. The dosage of compound 4 in each group was uniformly 10 mmol. Formamidine acetate was used for formamidine salt, and CuI was used for Cu(I) salt.
[0035] Example number
1-1
1-2
1-3
1-4
1-5
1-6
Dosage of formamidine acetate / mmol
15
15
15
15
15
15
L-proline dosage / mmol
2
2
2
2
2
2
CuI / mmol ...
Embodiment 2
[0038] Embodiment 2: the preparation of compound 2
[0039] The formula is as follows (compound 1 is 3-hydroxy-4-methoxybenzoic acid):
[0040] Material name
Feeding amount
Compound 1
10gram
100mL
[0041] concentrated sulfuric acid
20mL
[0042] operation process
[0043] Add compound 1 and methanol into the reaction flask, stir at room temperature, add concentrated sulfuric acid dropwise, and heat to reflux. After the reaction is complete, cool the solution to room temperature, neutralize with saturated aqueous sodium bicarbonate solution, concentrate to remove methanol, filter and wash with water to obtain Compound 2 (methyl 3-hydroxy-4-methoxybenzoate).
Embodiment 3
[0044] Embodiment 3: the preparation of compound 3,
[0045] The ratio is as follows:
[0046] Material name
[0047] Add the compound 2 and acetone obtained according to the method of Example 2 into the reaction flask, stir at room temperature, NBS in batches, and heat to reflux. After the reaction is completed for about 1 hour, cool the solution to room temperature, add saturated aqueous sodium bicarbonate solution, and concentrate Acetone was removed, and compound 3 was obtained by filtering and washing with water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com