Method for synthesizing iminostilbene
A technology of iminostilbenes and synthetic methods, applied in the direction of organic chemistry, can solve the problems of heavy pollution, long steps, low yield, etc., and achieve the effect of optimizing reaction conditions and shortening reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
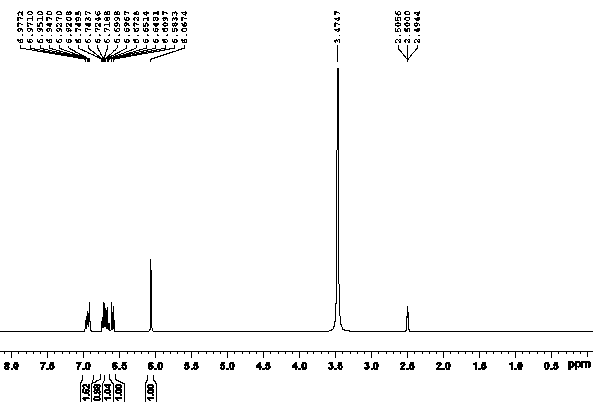
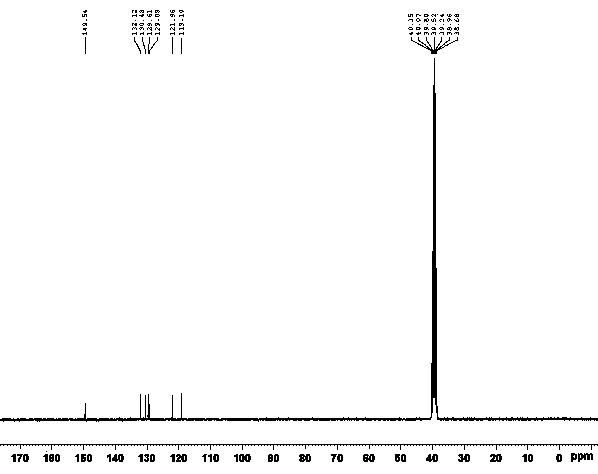
[0027] Under argon protection, add 0.10g (0.90mmol) of L-proline, 1.66g (12mmol) of potassium carbonate, 0.17g (0.30mmol) of cuprous iodide, and 1.30g (6mmol) of o-iodotoluene into a 100mL four-necked flask ), DMSO 9mL, stirred, heated to 90°C, added dropwise 0.64g (6mmol) of o-toluidine, reacted for 48-96 hours, followed by TLC. After the reaction was completed, cool to room temperature, pour into 14 mL of water, extract with ethyl acetate (3×30 mL), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill the filtrate under reduced pressure to obtain a brown solid, add Petroleum ether (2 mL) was dissolved, filtered to obtain a yellow solid, and finally recrystallized from toluene to obtain 0.24 g of a golden yellow solid, with a yield of 20.72% and a purity of ≥98%.
Embodiment 2
[0029] Under argon protection, add 0.17g (1.50mmol) of L-proline, 1g (18mmol) of potassium hydroxide, 0.13g (0.90mmol) of cuprous bromide, and 1.54g (9mmol) of o-bromotoluene into a 100mL four-necked flask. ), DMSO 15mL, stirred, heated to 100°C, added o-toluidine 0.64g (6mmol) dropwise, reacted for 48-96 h, followed by TLC. After the reaction was completed, cool to room temperature, pour into 22.50 mL of water, extract with ethyl acetate (3×30 mL), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill the filtrate under reduced pressure to obtain a brown solid. Add petroleum ether (2 mL) to dissolve, filter to obtain a yellow solid, and finally recrystallize with toluene to obtain 0.28 g of a golden yellow solid, with a yield of 24.17% and a purity of ≥98%.
Embodiment 3
[0031] Under argon protection, add 0.14g (1.20mmol) of L-proline, 0.82g (15mmol) of sodium methoxide, 0.12g (0.60mmol) of cuprous iodide, and 1.57g (7.20mmol) of o-iodotoluene into a 100mL four-neck flask. mmol), DMSO 12mL, stirred, heated to 100°C, 0.64g (6mmol) of o-toluidine was added dropwise, reacted for 48-96 hours, followed by TLC. After the reaction was completed, cool to room temperature, pour into 18mL water, extract with ethyl acetate (3×30mL), combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and distill the filtrate under reduced pressure to obtain a brown solid, add Petroleum ether (2 mL) was dissolved and filtered to obtain a yellow solid, which was recrystallized from toluene to obtain 0.49 g of a golden yellow solid with a yield of 42.31% and a purity of ≥98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com