Preparation method of phytosterol cinnamate
A technology for phytosterol esters and phytosterols, which is applied in the field of preparing phytosterol derivatives, can solve the problems of poor water solubility and fat solubility of phytosterols, limited application scope and the like, and achieves the effects of convenient preparation, widening application scope and improving fat solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
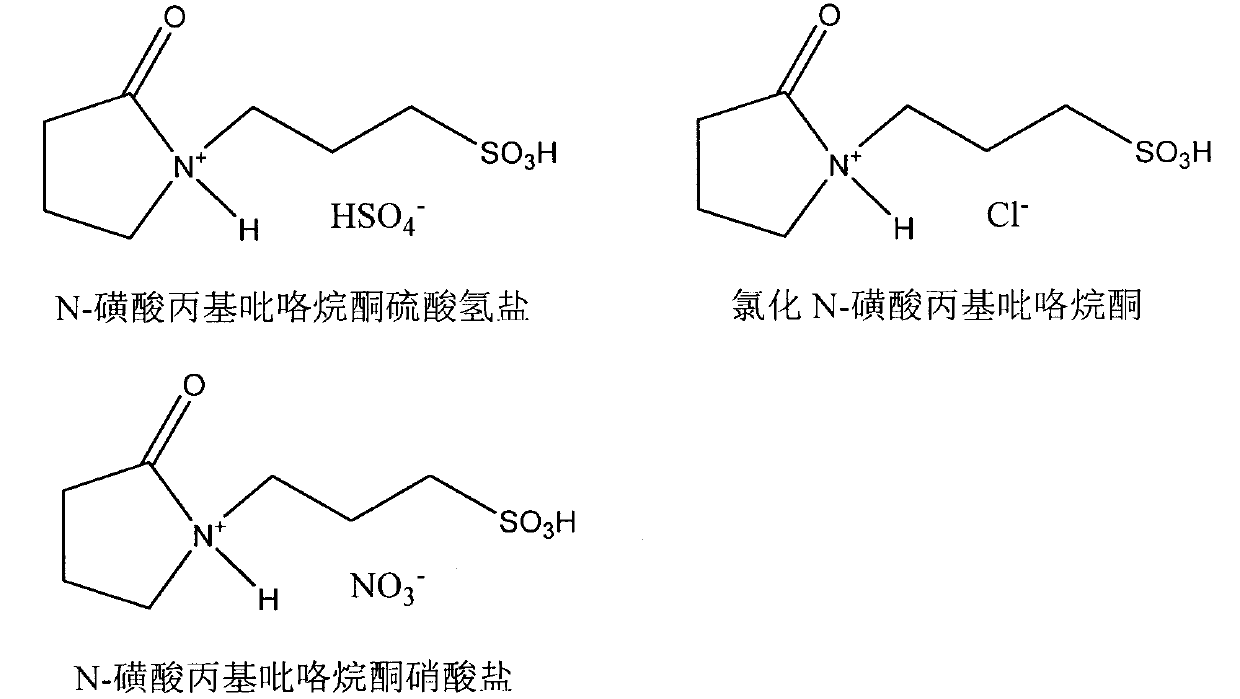
[0024] Add 0.2056g (0.5mmol) stigmasterol and 0.1480g (1mmol) cinnamic acid in the reaction flask with branch tube, feed N 2 After melting completely at 120°C under magnetic stirring, add 0.0038g (0.0125mmol) N-sulfonic acid propylpyrrolidone bisulfate and 0.0073g (0.0125mmol) cerous trifluoromethanesulfonate composite catalyst, at 120°C The reaction was incubated for 4 hours, and the reaction progress was detected by HPLC and TLC. After the reaction, the crude stigmasteryl cinnamate product was obtained, which was separated and purified by column chromatography to obtain the stigmasteryl cinnamate product, and its structure was identified by infrared, mass spectrometry and nuclear magnetic resonance spectrum analysis.
[0025] Stigmasteryl cinnamate: HPLC retention time (min) 7.345; IR (v, cm -1 )2942, 2866(C=C-H), 1710(C=O), 1640, 1454(C=C); MS m / z565.5(M + +Na), 475.4 (M + -C 7 h 6 +Na); 1 H NMR (400MHz, CDCl 3 , δ, ppm) 0.71 (3H, s, 18-H), 0.80 (6H, d, J=7.2Hz, 26-2...
Embodiment 2
[0027] Add 0.4102g (1mmol) stigmasterol and 0.1480g (1mmol) cinnamic acid in the reaction flask with branch tube, pass into N 2After melting completely at 160°C under magnetic stirring, add 0.0049g (0.02mmol) of N-sulfonic acid propylpyrrolidone chloride and 0.0117g (0.02mmol) of cerous trifluoromethanesulfonate composite catalyst, and keep warm at 160°C The reaction was carried out for 6 h, and the reaction progress was detected by HPLC and TLC. After the reaction, the crude product of stigmasteryl cinnamate was obtained, which was separated and purified by column chromatography to obtain the product of stigmasteryl cinnamate, and its structure was identified by infrared, mass spectrometry and nuclear magnetic resonance spectrum analysis, the data of which were the same as in Example 1.
Embodiment 3
[0029] Add 0.4082g (1mmol) phytosterol and 0.222g (1.5mmol) cinnamic acid in the reaction bottle with branch tube, pass into N 2 After melting completely at 140°C under magnetic stirring conditions, add 0.0041g (0.015mmol) N-sulfonic acid propylpyrrolidone nitrate and 0.0088g (0.015mmol) cerous trifluoromethanesulfonate composite catalyst, at 140°C The reaction was incubated for 3 h, and the reaction progress was detected by HPLC and TLC. After the reaction, the crude phytosteryl cinnamate product is obtained, which is separated and purified by column chromatography to obtain the phytosteryl cinnamate product, and its structure is identified by infrared, mass spectrometry and nuclear magnetic resonance spectrum analysis.
[0030] Cinnamic acid phytosterol ester: HPLC retention time (min) 6.321 ~ 8.159; IR (v, cm -1 ) 2940, 2866 (C=C-H), 1711 (C=O), 1639, 1450 (C=C); MS m / z553.4 (camesterol M + +Na), 565.5 (stigmasterol M + +Na), 567.5 (β-sitosterol M + +Na), 463.3 (cameste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com