Huperzine A micro-emulsion type cataplasm
A technology of huperzine A and babu agent, applied in the field of medicine, can solve the problems of limited percutaneous penetration of drugs, harsh growth environment of Melaleuca tower, low bioavailability, etc., so as to improve the penetration rate of percutaneous and reduce mining. , the effect of increasing the penetration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of huperzine A microemulsion cataplasm
[0021] Huperzine A 0.1g
[0022] Peanut oil 10g
[0023] Polyoxyethylene hydrogenated castor oil 10g
[0024] Poloxamer 5g
[0025] Polyethylene glycol stearate 5g
[0026] Macrogol 400 5g
[0027] 1-2 propylene glycol 5g
[0028] Glycerin 5g
[0029] Laurocaprazine 7g
[0030] Carbomer U10 30g
[0031] Povidone 8g
[0032] Sodium Carboxymethyl Cellulose 20g
[0033]Aluminum glycylate 5g
[0034] Distilled water 300ml
[0035] Dissolving huperzine A into peanut oil, then adding polyoxyethylene hydrogenated castor oil, poloxamer and polyethylene glycol stearate, stirring and mixing to form the drug-containing internal phase. At room temperature, use a constant temperature magnetic stirrer to slowly add water to the drug-containing inner phase while stirring, at a rate of 300r.min -1 Stir at a constant speed until clear and transparent to prepare 100 g of transparent huperzine A microemulsion....
Embodiment 2
[0036] Embodiment 2: Preparation of huperzine A microemulsion cataplasm
[0037] Huperzine A 0.1g
[0038] Castor oil 20g
[0039] Poloxamer 10g
[0040] Lecithin 4g
[0041] Pluronic F-68 2g
[0042] Tween-80 8g
[0043] Macrogol 400 5g
[0044] Lauroazepam 10g
[0045] Oleic acid 10g
[0046] Glycerin 6g
[0047] Carbomer 934 25g
[0048] Sodium polyacrylate 15g
[0049] Tragacanth Gum 10g
[0050] Aluminum glycylate 6g
[0051] Distilled water 350ml
[0052] Stir and mix huperzine A, castor oil, poloxamer, lecithin, Tween-80, and pluronic F-68 to form a drug-containing inner phase. At room temperature, use a constant temperature magnetic stirrer to slowly add water to the drug-containing inner phase while stirring, at a rate of 300r.min -1 Stir at a constant speed until clear and transparent to prepare 150 g of transparent huperzine A microemulsion. Mix polyethylene glycol 400, laurocapram, oleic acid, and glycerin as phase A; mix carbomer 934, sodium polyacr...
Embodiment 3
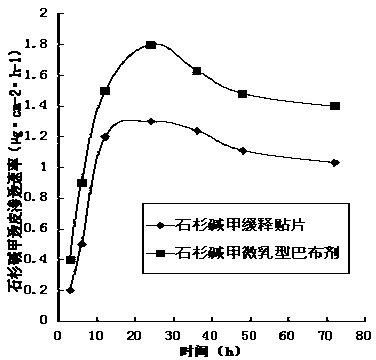
[0053] Example 3: Study on the transdermal penetration rate of huperzine A microemulsion cataplasm in human isolated skin
[0054] The Franz diffusion cell was used for skin penetration test, the skin was fixed at the mouth of the diffusion cell, and the stratum corneum was facing outward, and the same dose of huperzine A microemulsion cataplasm (prescription 1) or sustained-release patch was applied to the stratum corneum On the upper layer, the receiving medium is a 40% polyethylene glycol 400 saline solution by mass, and the effective area of the pool mouth is about 3.1cm 2 , with magnetic particles at 400r.min -1 Stirring at a constant speed, the diffusion tank is kept at a constant temperature of 37°C with a water bath jacket, samples are taken at the specified time, and an equal amount of receiving solution is added after sampling. The concentration of huperzine A in the permeate was determined by HPLC. The cumulative penetration Q at different times is plotted again...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com