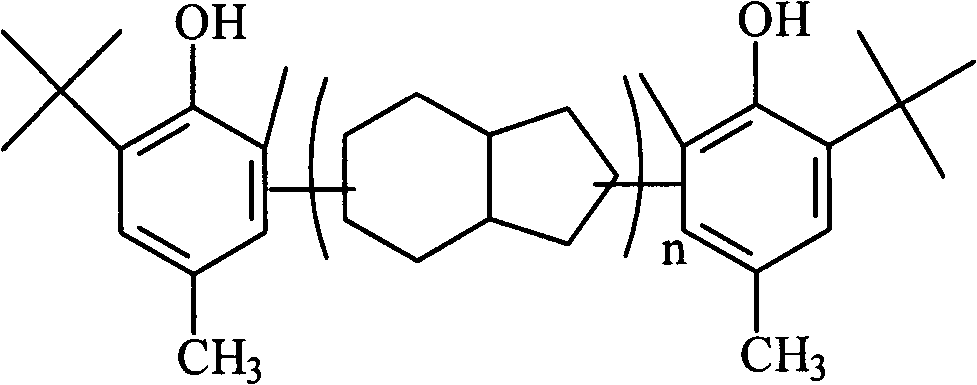
Preparation method for polymerization-type asymmetric hindered phenol anti-oxidant resins
A technology of asymmetric hindered phenol and antioxidant, which is applied in the field of preparing polymerized asymmetric hindered phenol antioxidant resin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 500ml reactor equipped with a stirrer, reflux condenser, feeding device and nitrogen protection device, add 400g toluene, 100g o-tert-butyl-p-cresol, 10g strong acid resin catalyst, replace with nitrogen, and heat to 83°C 120g of dicyclopentadiene was added dropwise with a pump, and the dropwise addition time was 30min. After the dropwise addition, the temperature was raised to 113°C. After 3.5h of reaction, 1.5g of phosphoric acid, 9g of alkylation catalyst were added, and the reaction temperature was 85°C, and methanol was added dropwise 30g, dripping time 60min, react for 120min after the completion of the dropwise addition, remove the resin catalyst by filtration after the reaction, wash with alkali and water until neutral, remove oligomers by steam stripping, and distill under reduced pressure after the solvent and unreacted raw materials, distill After cooling to 206° C., 136 g of the product was obtained, and the calculated product yield was 79.2%. The produ...
Embodiment 2
[0021] In a 500ml reactor equipped with a stirrer, reflux condenser, feeding device and nitrogen protection device, add 400g toluene, 100g o-tert-butyl-p-cresol, 20g strong acid resin catalyst, replace with nitrogen, and heat to 90°C 160g of dicyclopentadiene was added dropwise with a pump, and the dropwise addition time was 80min. After the dropwise addition, the temperature was raised to 120°C. After 4h of reaction, 2g of phosphoric acid, 13.5g of alkylation catalyst were added, and the reaction temperature was 78°C, and 35g of methanol was added dropwise. , the dropwise addition time is 45min, after the dropwise addition, react for 100min, remove the resin catalyst by filtration after the reaction, wash with alkali and water to neutrality, remove oligomers by steam stripping, and distill under reduced pressure after the solvent and unreacted raw materials, and distill to 152 g of the product was obtained after cooling at 239° C., and the calculated product yield was 82.4%. ...
Embodiment 3
[0023] In a 500ml reactor equipped with a stirrer, reflux condenser, feeding device and nitrogen protection device, add 600g toluene, 100g o-tert-butyl-p-cresol, 12g strong acid resin catalyst, replace with nitrogen, and heat to 95°C , Add 150g of dicyclopentadiene dropwise with a pump, and the dropping time is 90min. After the dropwise addition, the temperature is raised to 115°C. After 4.5h of reaction, 1.6g of phosphoric acid and 12g of alkylation catalyst are added. The reaction temperature is 80°C, and methanol is added dropwise. And ethanol molar ratio 1: 1 raw material 40g, dropwise time 40min, react 120min after dropwise, filter to remove resin catalyst after reaction, alkali wash and water wash to neutrality, water vapor stripping removes oligomer, solvent and untreated The reaction raw materials were then distilled under reduced pressure to 237° C., and 156 g of the product was obtained after cooling, and the product yield was calculated to be 83.5%. The product is a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com