Preparation method of antiviral medicine
A technology of antiviral drugs and products, which is applied in the direction of carboxylate preparation, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. Large-scale application and other issues, to achieve the effect of being suitable for industrial production, shortening the reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
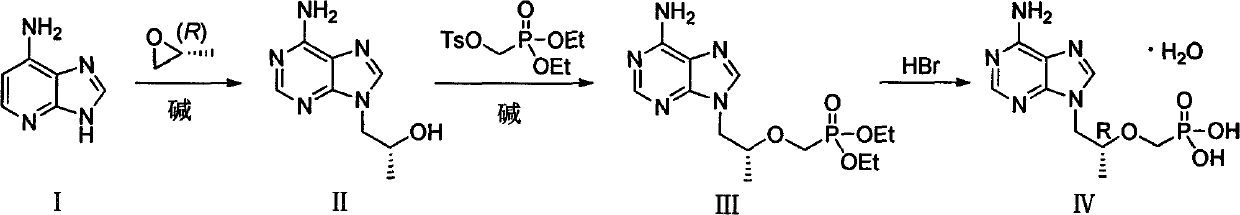
[0038] The first step: the preparation of (R)-1-(6-amino-9H-purin-9-yl)propyl-2-ol (II)
[0039] Add adenine (200.00g), sodium carbonate (7.80g) and DMF (1000ml) into a dry three-necked flask, stir at room temperature for 20min, then slowly add (R)-propylene oxide (150.50g), then react at 95°C 6h, the reaction system was cooled to room temperature, and slowly added toluene (2000ml), after the dropwise addition was completed, stirred at 0°C for 15h, filtered, the filter cake was washed with n-hexane, and dried in vacuo to obtain (R)-1-(6-amino- 9H-purin-9-yl)propyl-2-ol (II) 197.20 g, yield 69%.
[0040] 1 H NMR (400MHz, CDCl 3 )δ: 8.31(s, 1H), 7.81(s, 1H), 5.58(s, 2H), 4.29-4.19(m, 2H), 4.11(dd, J=13.8, 7.2Hz, 1H), 2.95(s , 1H), 1.28(d, J=6.4Hz, 3H).
[0041] MS(ESI):m / z[M+H] + calcd for C 8 h 11 N 5 O: 193.1; found: 194.2.
Embodiment 2
[0043] The first step: the preparation of (R)-1-(6-amino-9H-purin-9-yl)propyl-2-ol (II)
[0044] Add adenine (80.00g), sodium hydroxide 1.20g (60.70g) and DMF (500ml) in the dry three-necked flask, stir at room temperature for 20min, then slowly add (R)-propylene oxide (68.80g), and then React at 80°C for 5h, cool the reaction system to room temperature, and slowly add toluene (1050ml), after the dropwise addition, stir at 0°C for 16h, filter, wash the filter cake with n-hexane, and dry in vacuo to obtain (R)-1-(6 -Amino-9H-purin-9-yl)propyl-2-ol (II) 74.00 g, yield 65%.
Embodiment 3
[0046] Step 2: Preparation of (R)-diethyl(((1-(6-amino-9H-purin-9-yl)propyl-2-ol)oxy)methyl)phosphate (III)
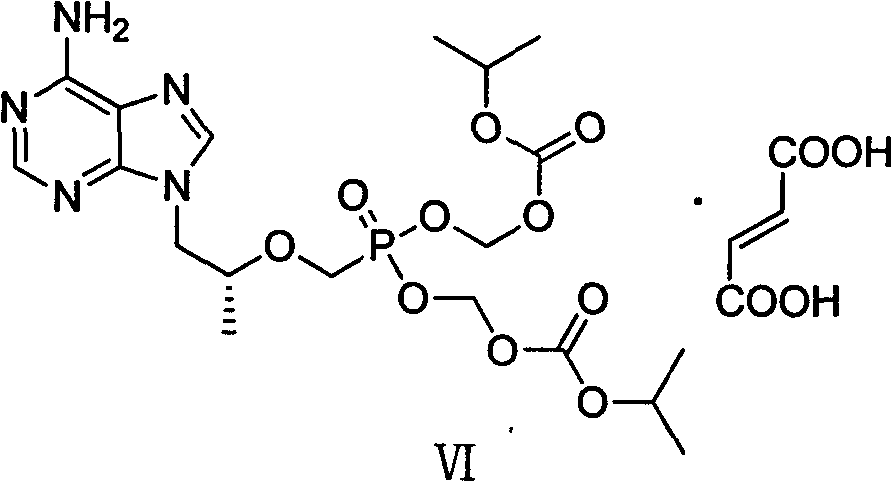
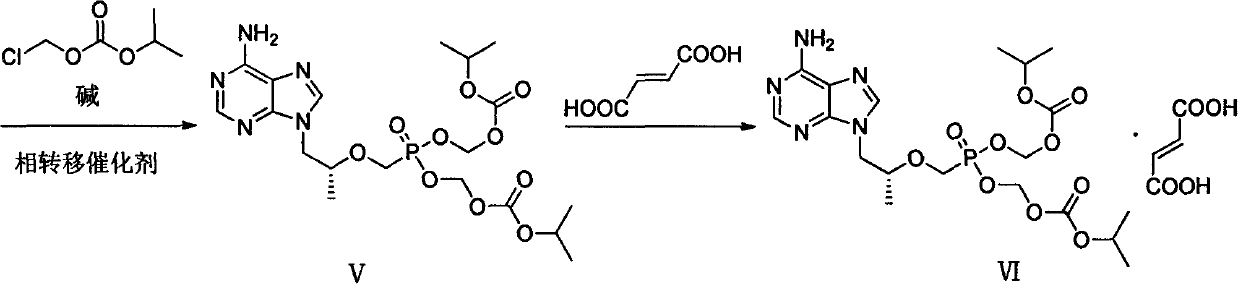
[0047] Add intermediate (II) (36.00g) and DMF (240ml) to a dry three-necked flask and stir to dissolve, add 18.37g of lithium tert-butoxide at 0°C, then react at room temperature for 2h, then slowly add (diethoxyphosphonyl ) 60.08g of methyl-4-methylbenzenesulfonate, reacted at 0°C for 12h, then added 9.19g of lithium tert-butoxide at the same temperature, stirred for 1h, then added (diethoxyphosphono)methyl-4- Toluenesulfonate (30.04g), stirred for 5h; then added 4.5g of lithium tert-butoxide, stirred for 1h, then added (diethoxyphosphono)methyl-4-methylbenzenesulfonate 15.02g, with After warm stirring for 12 hours, acetic acid was slowly added to the reaction system to adjust the pH to 6-7, and the mixture was concentrated under reduced pressure to obtain a light yellow oil. The yield of the crude product was calculated quantitatively, and it was directly put into th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com