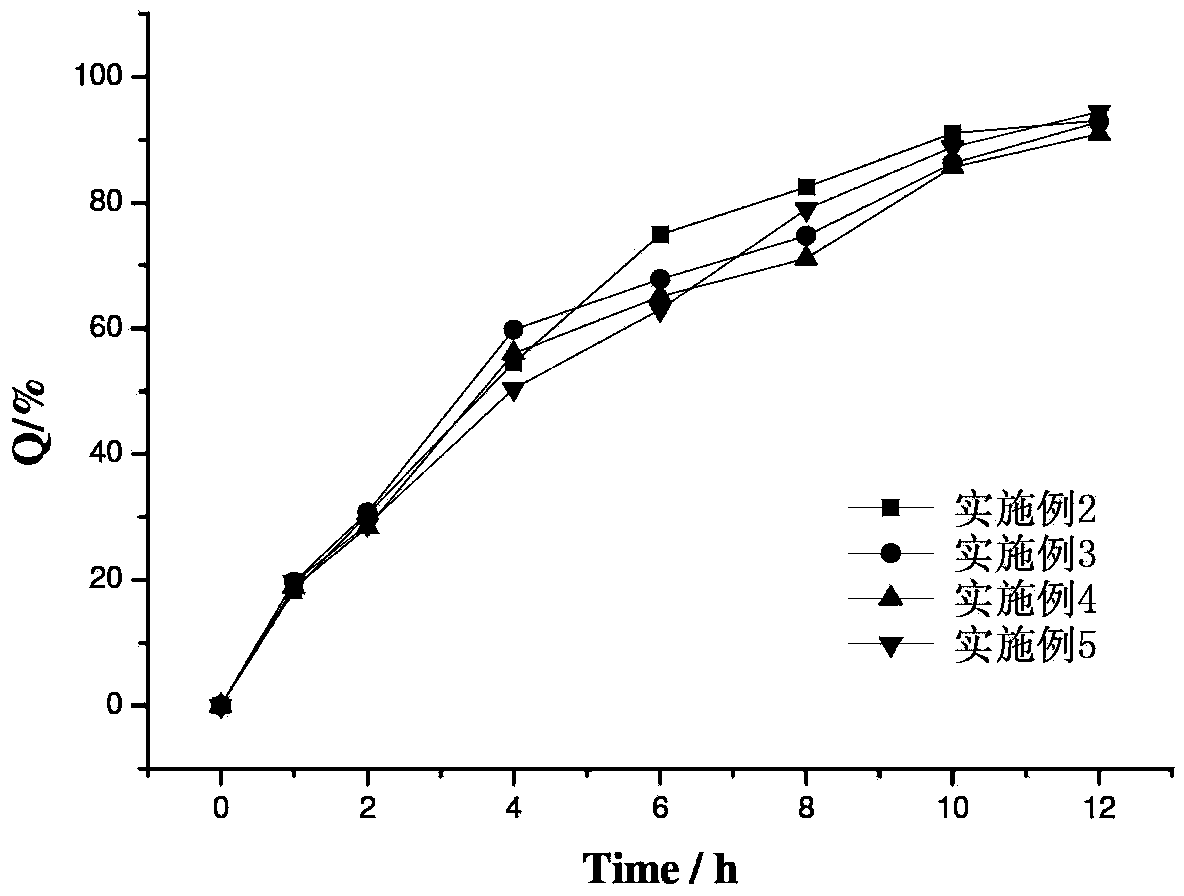
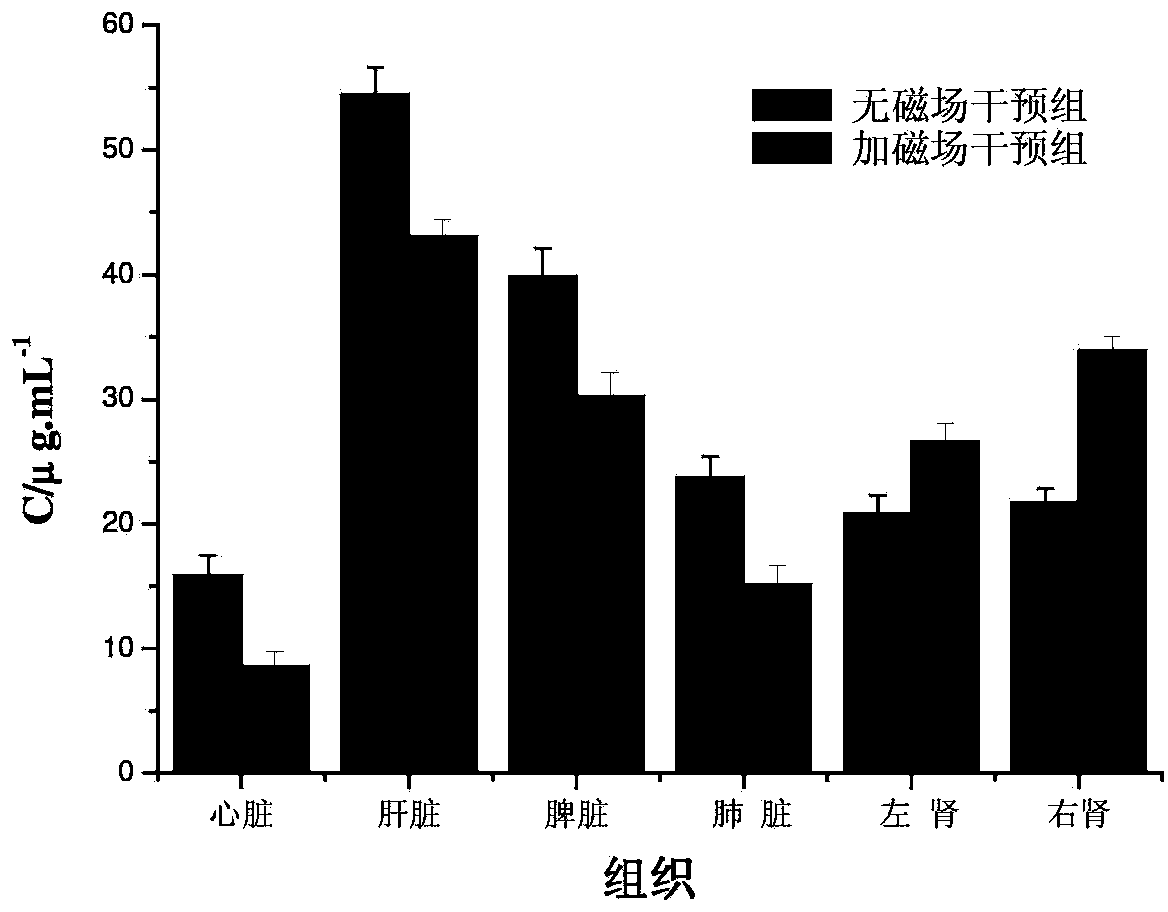
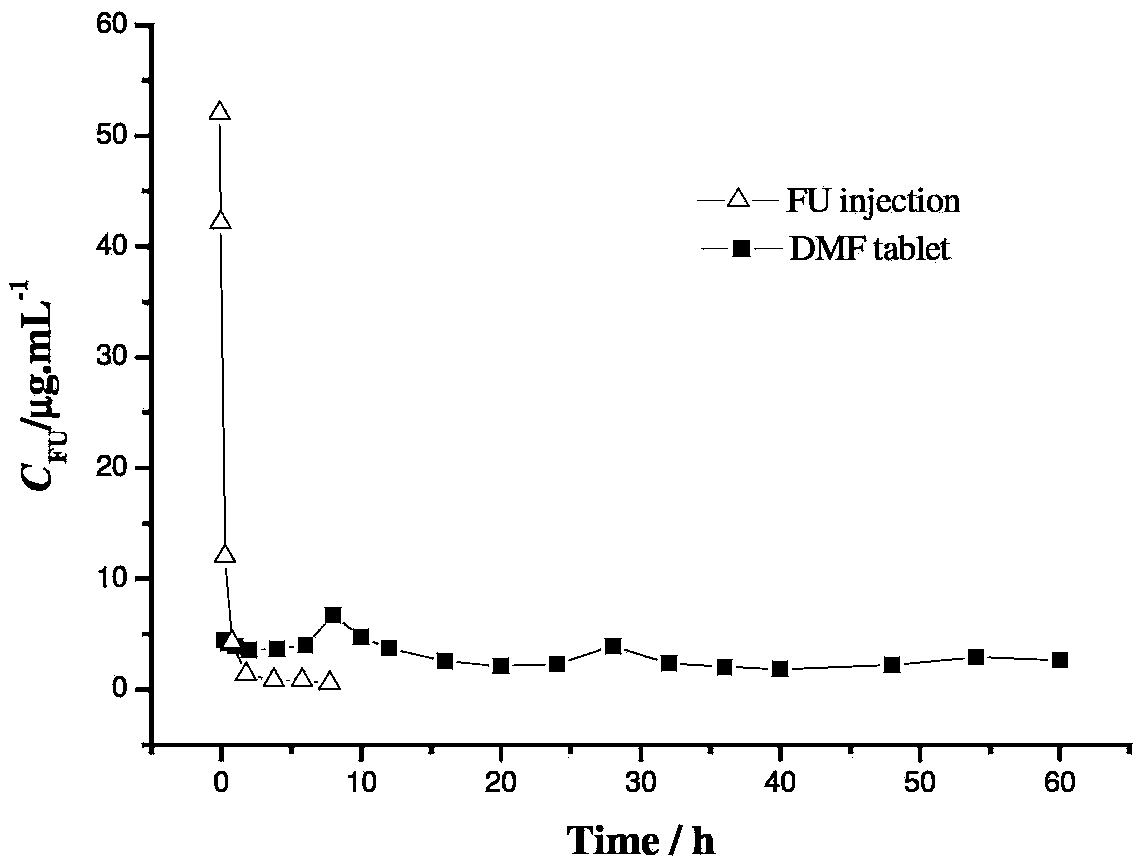
Dextran-MLDH-fluorouracil supermolecular assembly magnetic targeting sustained release tablet
A technology of fluorouracil and dextran, which is applied in the field of "dextran-MLDH-fluorouracil" supramolecular assembly type magnetic targeting sustained-release tablets, can solve the problems of short half-life, strong toxic and side effects, and fast metabolism, so as to prolong the half-life in vivo and improve biological Utilization, reduction of acute toxicity level and effect of plasma protein binding rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Example 1 The DMF magnetic drug carrier with a fluorouracil content of 20 to 40% was synthesized by the "co-precipitation intercalation-in-situ composite-solvent conversion" method. The specific operation process is as follows:
[0032] Accurately weigh 29.82g FeCl 2 4H 2 O and 13.52 g FeCl 3 ·6H 2 O, add to 500mL N 2 Protect the reactor, add 150mL distilled water 2 Stir to dissolve. Weigh 26.0g of fluorouracil, dissolve it with 100mL of 2M NaOH solution, add it to the mixed salt solution, and stir it evenly. Use 2M NaOH solution as precipitating agent, control the coprecipitation terminal pH to 7.0; 2 Or Ar protection, constant temperature at 60°C and 300r·min -1 The precipitation reaction was completed under the condition of magnetic stirring, and the crystallization was carried out for 45 minutes. Weigh 13.52g dextran, dissolve it with 50mL double-distilled water, add it to the reactor, and react in situ for 35min. Change the solvent with ethanol that was fro...
Embodiment 2
[0033] Embodiment 2 takes the DMF prepared in Example 1 as the drug carrier, and presses the tablet according to the following prescription, and the specific operations are as follows:
[0034] DMF drug carrier (powder)
[0035] Firstly, DMF is solid-phase ground into powder, passed through 80 mesh sieve, hydroxypropyl methylcellulose and chitosan respectively passed through 80 mesh sieve, lactose and magnesium stearate respectively passed through 100 mesh sieve; Each powdery substance is fully mixed by an equal amount incremental method; finally, an appropriate amount of the mixed powder is taken, and the matrix tablet is directly made into a matrix tablet on a single-punch tablet machine with a shallow concave die by the powder direct compression method.
[0036] Embodiment 3 uses the processing method of embodiment 2, compresses tablet by following prescription:
[0037] DMF drug carrier (powder)
[0038] Processing method is with embodiment 2.
[0039]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Magnetic field strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com