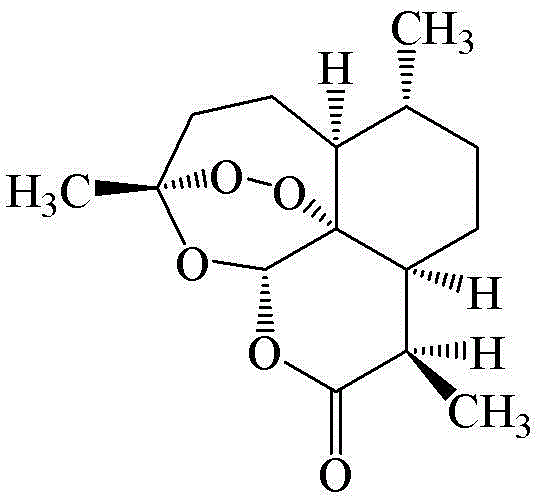
Method for preparing antimalarial active compound artemisinin through direct column chromatography
A column chromatography, artemisinin technology, applied in organic chemistry and other directions, can solve the problems of high cost, many links, time-consuming and labor-intensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] Example. Artemisinin was prepared using the following method and operating conditions. Specifically: the raw materials of Artemisia annua leaves are naturally dried, pulverized, and passed through a 100-mesh sieve. Take 0.5 g of Artemisia annua leaf powder (100 mesh), mix it with 0.5 g of silica gel (80-100 mesh) and grind it evenly, and cover it on a glass chromatography column (30×1 cm) filled with 2.0 g of silica gel (80-100 mesh). ), elute with petroleum ether: ethyl acetate (85: 15) as the mobile phase, and collect fractions (4 ml is a fraction). Fractions 3-6 were concentrated under reduced pressure and crystallized with petroleum ether to obtain 6 mg of white needle-like crystals of artemisinin. The yield was 1.2 percent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com