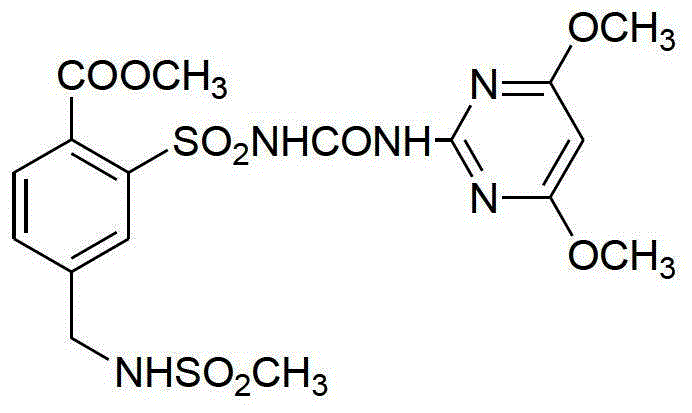
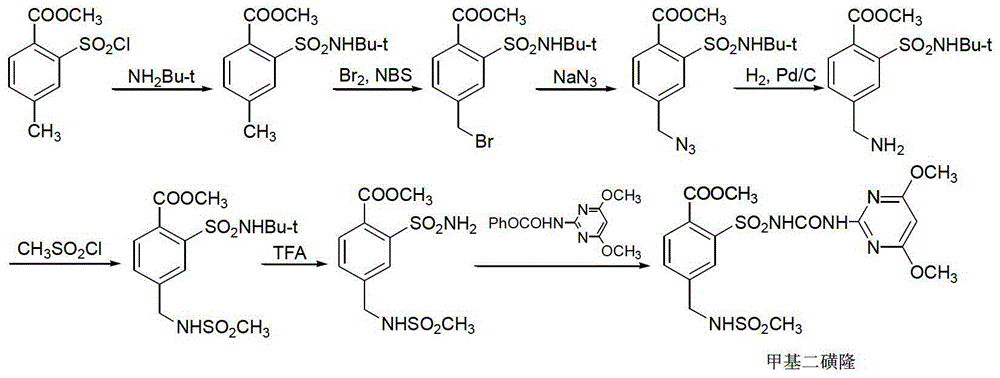
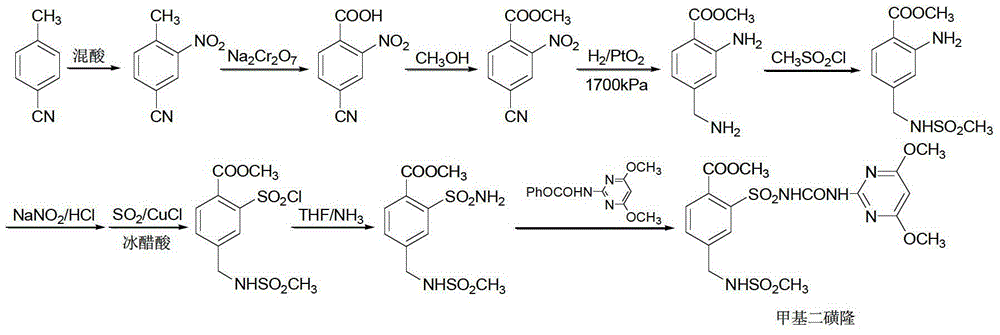
Mesosulfuron-methyl synthetic method
The technology of methyldisulfuron-methyl and the methyldisulfuron-methyl is applied in the field of preparation of organic compounds, can solve the problems of complicated synthesis steps, high production cost, poor practicability, etc. Practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthetic method of methylsulfuron-methyl, the steps are as follows:
[0049] 1) Preparation of 2-methyl-5-aminocarbonylbenzenesulfonamide (2)
[0050] Add 13.6g p-toluic acid (0.1mol), 60.0mL 1,2-dichloroethane and 14.5mL thionyl chloride (0.2mol) into the reaction flask, stir to dissolve, add 25.3mL chlorosulfonic acid (0.4 mol), after the addition, the temperature was raised to 60°C. After reacting for 5 hours, the solvent, thionyl chloride and chlorosulfonic acid were distilled off under reduced pressure, and the residue was cooled and added dropwise to 100mL of ammonia water, the solid precipitated, filtered, washed with water until neutral, and dried to obtain (2) 18.2g, yield: 85.2%.
[0051] 2) Preparation of 5-aminocarbonyl saccharin (3)
[0052] Add 21.4g (2) (0.1mol) and 40mL98% concentrated sulfuric acid into the reactor, stir and cool to 0~5°C, add 88.4g potassium dichromate (0.3mol) and 60mL98% concentrated sulfuric acid dropwise within 0.5h of the...
Embodiment 2
[0064] The synthetic method of methylsulfuron-methyl, the steps are as follows:
[0065] 1) Preparation of 2-methyl-5-aminocarbonylbenzenesulfonamide (2)
[0066] Add 13.6g p-toluic acid (0.1mol), 50.0mL 1,2-dichloroethane and 21.7mL thionyl chloride (0.3mol) into the reaction flask, stir to dissolve, add 19.0mL chlorosulfonic acid (0.3 mol), after the addition, the temperature was raised to 60°C. After reacting for 5 hours, the solvent, thionyl chloride and chlorosulfonic acid were distilled off under reduced pressure, and the residue was cooled and added dropwise to ammonia water (ice bath). The solid precipitated, filtered, washed with water until neutral, and dried to obtain (2) 17.6g. Yield: 82.2%.
[0067] 2) Preparation of 5-aminocarbonyl saccharin (3)
[0068] Add 21.4g (2) (0.1mol) and 40mL98% concentrated sulfuric acid into the reactor, stir and cool to 0~5°C, add 89.4g sodium dichromate (0.3mol) and 50mL98% concentrated sulfuric acid dropwise within 0.5h The mix...
Embodiment 3
[0080] The synthetic method of methylsulfuron-methyl, the steps are as follows:
[0081] a. Add the raw materials p-toluic acid, organic solvent I and thionyl chloride into the reactor, stir to dissolve, add chlorosulfonic acid at room temperature, react at 30°C for 6 hours, and distill off the unreacted raw materials under reduced pressure , the raffinate was cooled to room temperature, added dropwise to ammonia water, the solid was precipitated, filtered, washed with water to pH 7-8, and dried to obtain 2-methyl-5-aminocarbonylbenzenesulfonamide;
[0082] The molar ratio of p-toluic acid: chlorosulfonic acid: thionyl chloride: organic solvent I is 1:3:2:5;
[0083] The ammonia water is NH 3 The mass percentage content is 25% ammoniacal liquor, and the mass ratio of described raffinate and ammoniacal liquor is 1:10;
[0084]b. Add 2-methyl-5-aminocarbonylbenzenesulfonamide and concentrated sulfuric acid into the reactor, stir and cool to 0-10°C, add oxidant and concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com