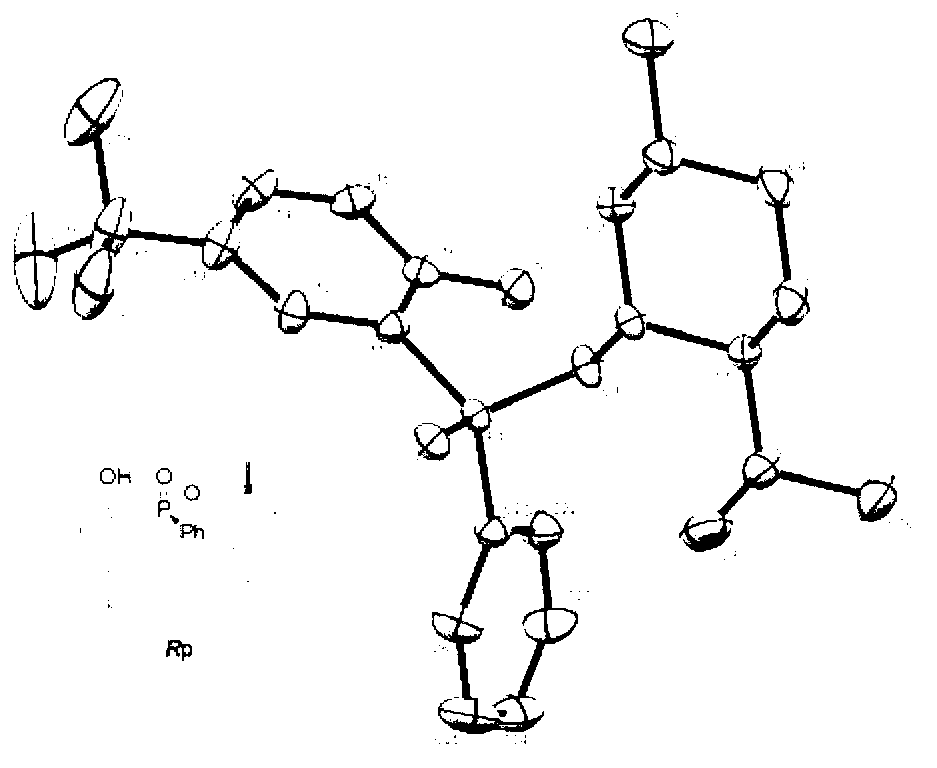
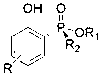Phenol derivative containing (Rp)-2-chiral phosphinate substituent and preparation method thereof
A phosphinate and substituent technology, applied in the field of asymmetric catalysis synthesis, can solve the problems of difficult recovery and utilization of chiral resolving agents, low activity and selectivity of resolving agents, cumbersome experimental steps, etc., and achieves good industrial application. Prospects, cheap catalysts, and the effect of simple and easy methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 2.80 g (10 mmol) ( R p)-Phenylphosphinate-(-)-menthol ester, 1.034 g (11 mmol) phenol, 50 mL carbon tetrachloride and 2.78 mL (20 mmol) triethylamine were added to a round bottom flask under nitrogen atmosphere , stirred at room temperature for 12 h to obtain the target product R p-(-)-menthol- O -Phenyl-phenylphosphinate, the yield is 99%; take 1.86 g (5 mmol) R p-(-)-menthol- O -Phenyl-phenylphosphinate, added to 25 mL tetrahydrofuran solution under nitrogen atmosphere, and heated at -78 o Slowly add this solution dropwise to the mixed solution of lithium diisopropylamide (5.5 mmol)-tetrahydrofuran (25 mL) at ℃, and then under the condition of stirring from minus 78 o C was warmed up to room temperature and continued to react for 6 h to obtain the target product R p-(-)-menthol-2-hydroxyphenyl-phenylphosphinate in 99% yield.
[0034]
Embodiment 2
[0036] Take 2.80 g (10 mmol) (R p)-Phenylphosphinate-(-)-menthol ester, 0.9494 g (10.1 mmol) phenol, 40 mL toluene, 10 mL carbon tetrachloride, and 6.52 g (20 mmol) cesium carbonate were added to the round bottom under nitrogen atmosphere In the flask, stir the reaction at room temperature for 12 h to obtain the target product R p-(-)-menthol- O -Phenyl-phenylphosphinate, the yield is 91%; take 1.86 g (5 mmol) R p-(-)-menthol- O -Phenyl-phenylphosphinate, added to 25 mL tetrahydrofuran solution under nitrogen atmosphere, and heated at -78 o Slowly add this solution dropwise to the mixed solution of lithium diisopropylamide (6.5 mmol)-tetrahydrofuran (25 mL) at ℃, and then under the condition of stirring from minus 78 o C was warmed up to room temperature and continued to react for 6 h to obtain the target product R p-(-)-menthol-2-hydroxyphenyl-phenylphosphinate in 99% yield.
[0037]
Embodiment 3
[0039] Take 2.80 g (10 mmol) ( R p)-Phenylphosphinate-(-)-menthol ester, 1.015 g (10.8 mmol) phenol, 40 mL acetonitrile, 10 mL carbon tetrachloride and 4.24 g (20 mmol) potassium phosphate were added to the circle under nitrogen atmosphere Bottom flask, stirred at room temperature for 12 h to obtain the target product R p-(-)-menthol- O -Phenyl-phenylphosphinate, the yield is 82%; take 1.86 g (5 mmol) R p-(-)-menthol- O -Phenyl-phenylphosphinate, added to 25 mL tetrahydrofuran solution under nitrogen atmosphere, and heated at -78 o Slowly add this solution dropwise to the mixed solution of lithium diisopropylamide (7.5 mmol)-tetrahydrofuran (25 mL) at ℃, and then under the condition of stirring from minus 78 o C was warmed up to room temperature and continued to react for 6 h to obtain the target product R p-(-)-menthol-2-hydroxyphenyl-phenylphosphinate in 99% yield.
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com