Active attapulgite medicine and preparation method for same
An attapulgite and active technology, applied in the field of medicine, can solve the problems of unstable quality of attapulgite, unsuitable application of attapulgite to the pharmaceutical and food industries, unexpressed process parameters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
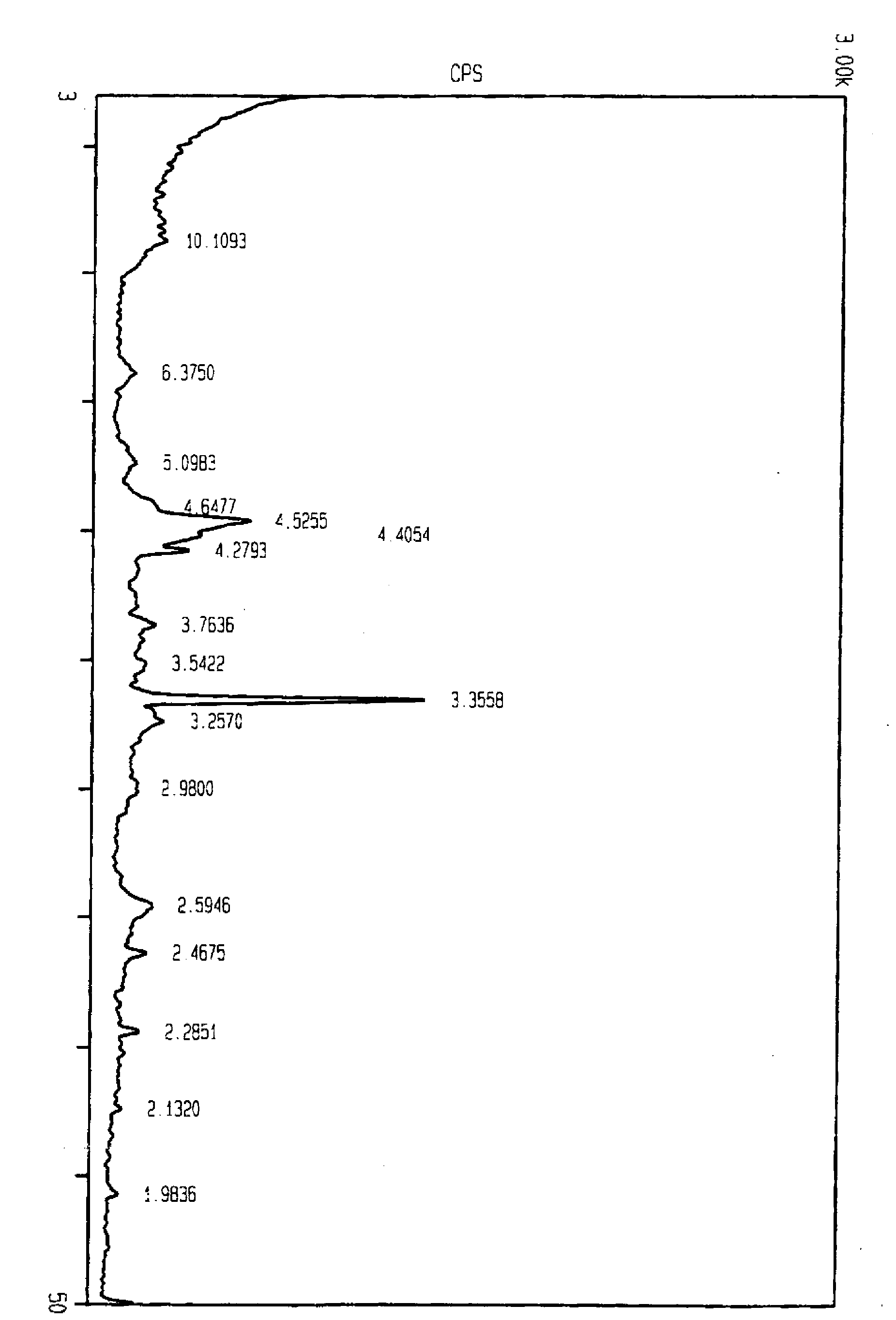
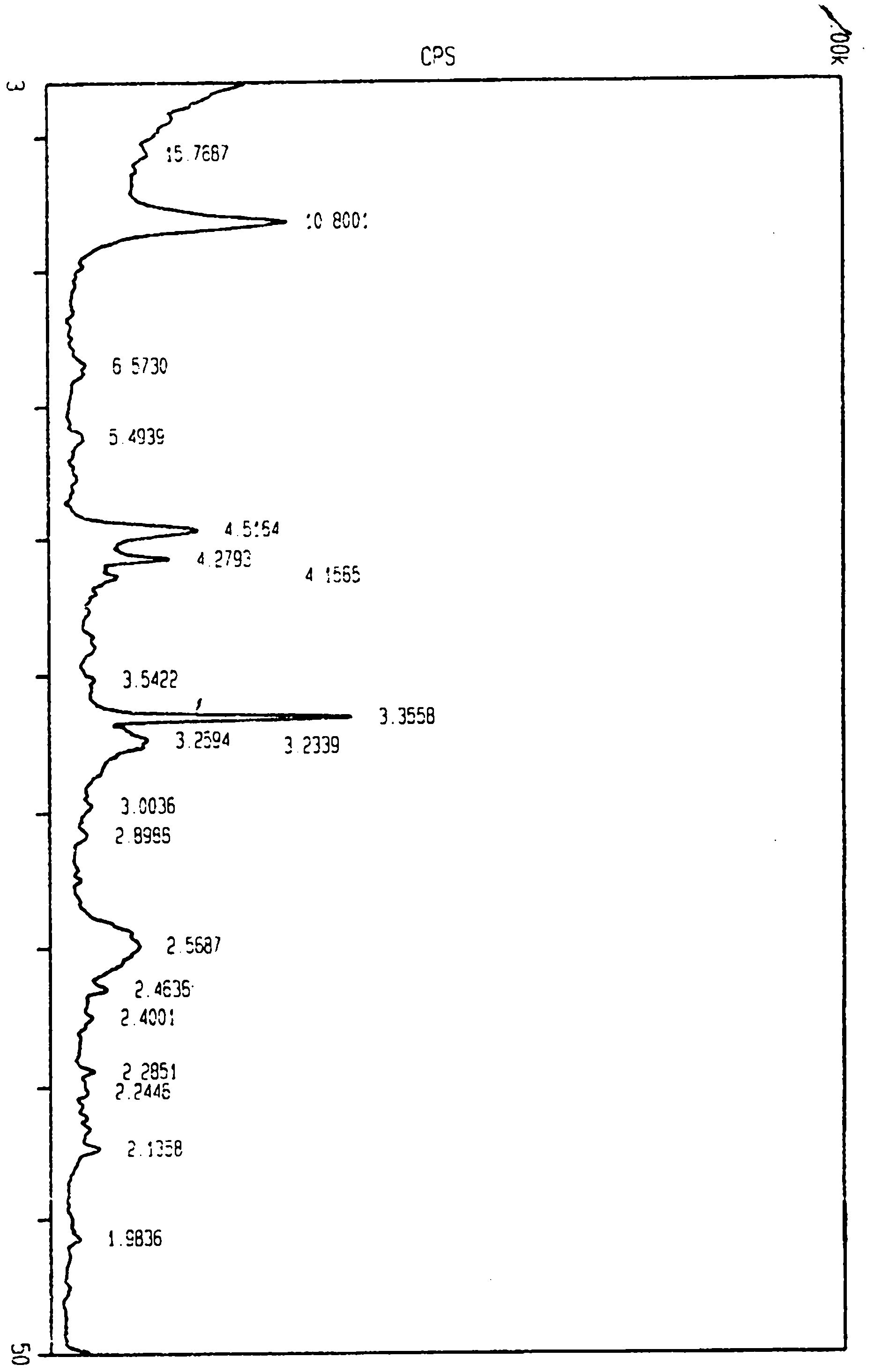
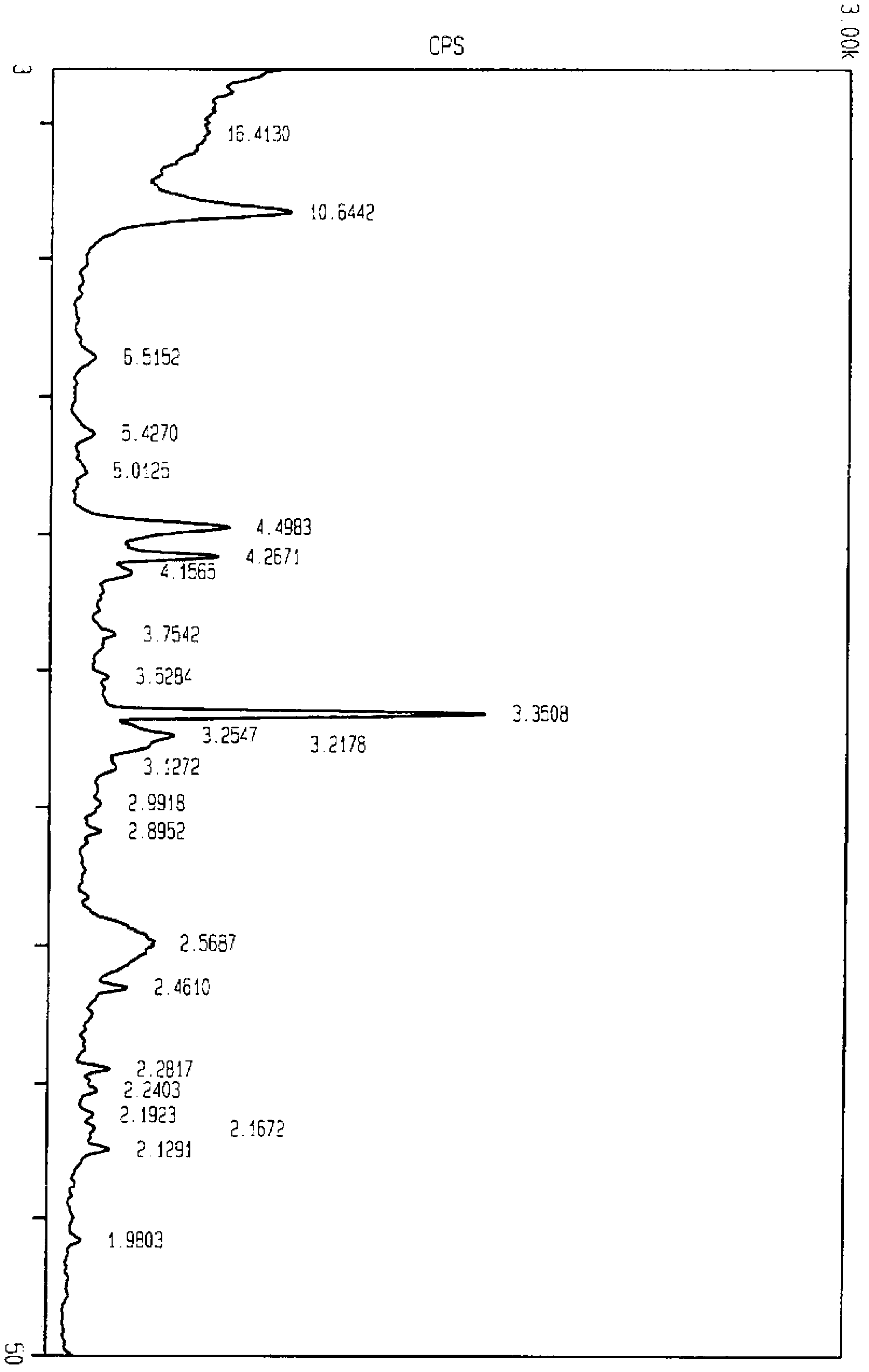
Image
Examples
Embodiment 1
[0085] Embodiment 1, an active attapulgite medicine for treating diarrhea and flatulence gas gastrointestinal diseases, the preparation method steps are as follows:
[0086] (1) Dry the attapulgite clay with a heated drum dryer, the drum speed is controlled at 20r / min, the drying temperature is 130°C, and dried in the dryer for 10h until the water content is 3.2wt%. ;
[0087] The dry product was soaked in purified water for 24 hours and then stirred. The mass ratio of the dried attapulgite clay to purified water was 1:10; and then dispersed in a disperser with a dispersing shaft speed of 1400r / min and a dispersing disc diameter of 500mm, the dispersion time is controlled within 15 minutes; after the dispersion is completed, pass through a 300-mesh sieve to obtain a coarse material liquid;
[0088] (2) Ultrasonic disperse the crude material liquid at 20°C for 50 minutes, and the ultrasonic frequency is 35KHz to obtain a suspoemulsion; keep the upper suspoemulsion, and then re...
experiment example 1
[0095] Experimental example 1. Acute toxicity test of active attapulgite
[0096] (1) Medicine: the active attapulgite powder prepared in Example 1, ready for use.
[0097] (2) Animals: Golden hamsters are of ordinary grade, provided by the Experimental Animal Center of Shandong University.
test approach —
[0098] (3) Test method - Kou's method:
[0099] The test steps are as follows:
[0100] Pre-test
[0101] Select 80 healthy adult rats (body weight 130g-150g) and mice (body weight 18g-22g) each (half male and half male). Select 20 rats from them, divide them into four groups randomly with 5 rats in each group, and do the pre-test. The feeding doses were 500, 5000, 10000, 20000 mg / kg body weight respectively. After oral gavage for 5 days, none of the animals died.
[0102] formal test
[0103] Set up the formal test dose group: choose healthy adult rats (body weight 130g-150g), mice (body weight 18g-22g), 70 each (half male and half female), and the rats are randomly divided into five groups with 14 rats in each group, and fed respectively. The doses are 5762, 8232, 11760, 16800 and 24000 (mg / kg body weight), five dose groups. The mice were randomly divided into five groups with 14 mice in each group, and the doses were 5931, 8472, 12103, 17290 and 24700 (mg / kg body weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
| Dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com