A method and application of rapid preparation of polyamino acid and its derivatives
A technology of polyamino acids and derivatives, applied in other methods of inserting foreign genetic materials, pharmaceutical formulations, bulk chemical production, etc., can solve problems such as quenching and chain transfer, harsh polymerization conditions, and large solvent consumption, and achieve solution High polarity, easy recycling, and no pollution cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
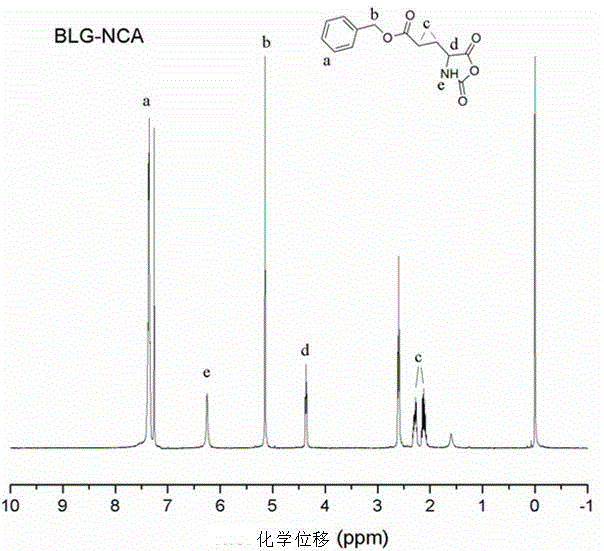
[0031] S1. Preparation of L-type α-benzyl glutamate: 30 g of L-glutamic acid and 28.9 g of benzyl alcohol were mixed in a 250 ml round bottom flask, then heated to 70 °C in an oil bath, and then slowly dropped into 55 The concentrated sulfuric acid with a mass fraction of g of 66% was heated and stirred at 70°C until the solution changed from turbid to completely clear, then the round bottom flask was placed under a rotary evaporator and kept in a water bath at 60°C for 4 hours, and then the obtained The viscous liquid was slowly poured into 250 ml of ice water containing 33 g of sodium bicarbonate without excess CO 2 After release, the ice-water mixture containing the precipitate was placed in a refrigerator at 4°C for 24 hours, then filtered, and the obtained filter cake was washed with absolute ethanol for 4 hours. The white precipitate was reprecipitated 4 times with deionized water. This side is also applicable to the preparation of benzyl aspartate.
[0032] S2...
Embodiment 2
[0041] S1. Preparation of L-type α-aspartic acid benzyl ester: same as Example 1.
[0042] S2. Preparation of L-type α-aspartic acid benzyl ester-N-carboxylic acid anhydride: same as Example 1.
[0043] S3. Synthesis of poly(benzyl L-aspartate): only the ionic liquid solvent is 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4), and other steps are the same as in Example 1. The specific experimental results are shown in the table below:
[0044]
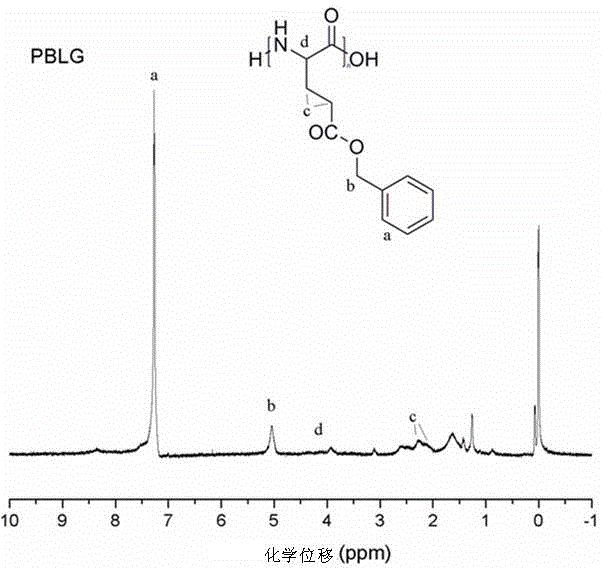
[0045] S4. Preparation of poly(L-aspartic acid) by deprotection of poly(L-aspartic acid benzyl ester): same as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com