Method for separating streptococcus pneumoniae in complicated substrate
A Streptococcus pneumoniae and complex matrix technology, applied in the biological field, can solve the problems of separation failure, increase in antibody spatial direction, cell extrusion, etc., to increase the chance of contact, realize cascade amplification, and stabilize the reaction solution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] experiment one:
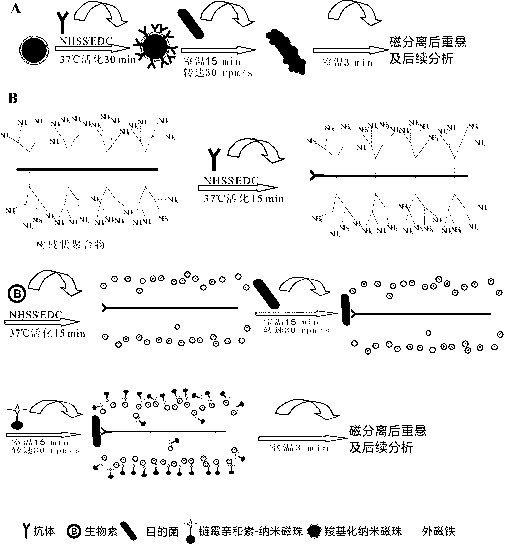
[0030]1. The dendritic polymer-antibody complex is prepared according to the following steps:
[0031] (1) Dissolve 3.6mg of dendrimer aminated polyamide-amine dendrimer in 2mL, 0.02M, pH6.5PBS, add 0.6mgNHSS, 0.4mgEDC, stir on a mixer at room temperature, activate 15min;
[0032] (2) Take 10.5mg SP The specific antibody was added to the above reaction solution, placed on a mixer at room temperature and stirred for 30 minutes;
[0033] (3) After the above solution was vacuum-dried, dissolved in deionized water, dialyzed in PBS and deionized water for 1 day; after the dialysis, the obtained solution was freeze-dried.
[0034] 2. The long-chain biotin-dendrimer-antibody complex is prepared according to the following steps:
[0035] (1) Dissolve 15mg of long-chain biotin, 3.6mg of NHSS, and 2.4mg of EDC in 2mL of 0.02MpH6.5PBS buffer;
[0036] (2) Add 0.55 mg of the dendrimer-antibody complex to the above solution, place it on a mixer at room temper...
Embodiment 2
[0040] Embodiment 2 enrichment effect experiment
[0041] (1) Take 1mL concentration as 10 4 cfu / mL Streptococcus pneumoniae in a 1.5mL sterile centrifuge tube, centrifuge at 12000rpm for 5min, discard the supernatant, and resuspend with an equal volume of sterile PBS solution.
[0042] (2) Enrichment and capture: Set up the technical solution group of the present invention (dendritic polymer group co-modified by Streptococcus pneumoniae antibody and long-chain biotin), nanomagnetic beads group modified by Streptococcus pneumoniae specific antibody, Streptococcus pneumoniae Specific antibody-modified micron magnetic bead group enriches target bacteria.
[0043] (3) After magnetic separation, pour the supernatant into a sterile centrifuge tube, and wash the isolated immunomagnetic beads with Streptococcus pneumoniae twice with PBST, mix well, and resuspend the immunomagnetic beads with 1 mL of sterile PBS solution. Magnetic bead complex.
[0044] (4) Capture rate calculation...
Embodiment 3
[0057] Example 3 Enrichment capture experiment
[0058] Conventional magnetic stand separation time is 30min, and all the other are with embodiment 2.
[0059] The catch rate of each group is as follows:
[0060] Capture efficiency of micron magnetic bead set modified with specific antibody of Streptococcus pneumoniae Capture efficiency of nano-magnetic bead set modified with specific antibody of Streptococcus pneumoniae Capture efficiency of dendrimers co-modified with Streptococcus pneumoniae antibody and long-chain biotin 46.5% 40.6% 90.9%
[0061] The experimental results show that compared with the separation of 3min in Example 2, when the separation time reaches 30min, the capture efficiency of the three groups has been improved, especially the capture efficiency of the nano-magnetic bead group modified by the specific antibody of Streptococcus pneumoniae is the most obvious. This indicated that the capture efficiency of the magnetic nanobeads gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com