Triarylsulfonium salt as well as preparation method and application thereof
A technology of triaryl and sulfonium salts, applied in the application field of triaryl sulfonium salts and their preparation, and UV-curable coatings, can solve the problems of small molecular weight, migration, and volatilization of benzophenone, and achieve good Reactivity, improvement of curing efficiency, high reactivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put a magnetic stirring bar, 4-benzoyl-4'-methyl-diphenyl sulfide (BMS) (1.0 mmol), and diphenyl iodine trifluoromethanesulfonate in a two-necked flask equipped with a condenser (1.2mmol) and copper acetate (0.1mmol) were added into the reactor, mixed evenly, and reacted for 30min in an oil bath at 130°C under solvent-free conditions. After cooling to room temperature, the mixture was sequentially separated by column chromatography using dichloromethane, ethyl acetate and ethanol as eluents to obtain a light yellow viscous product (I).
[0022]
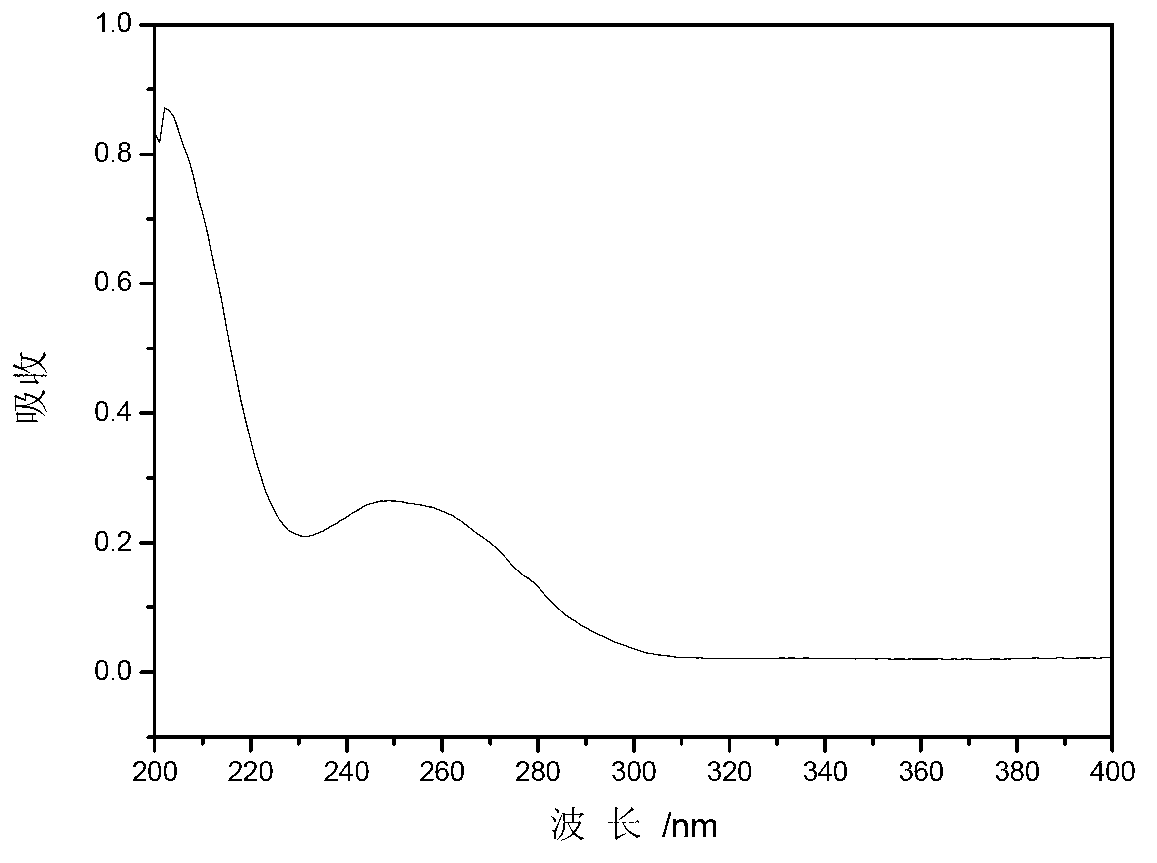
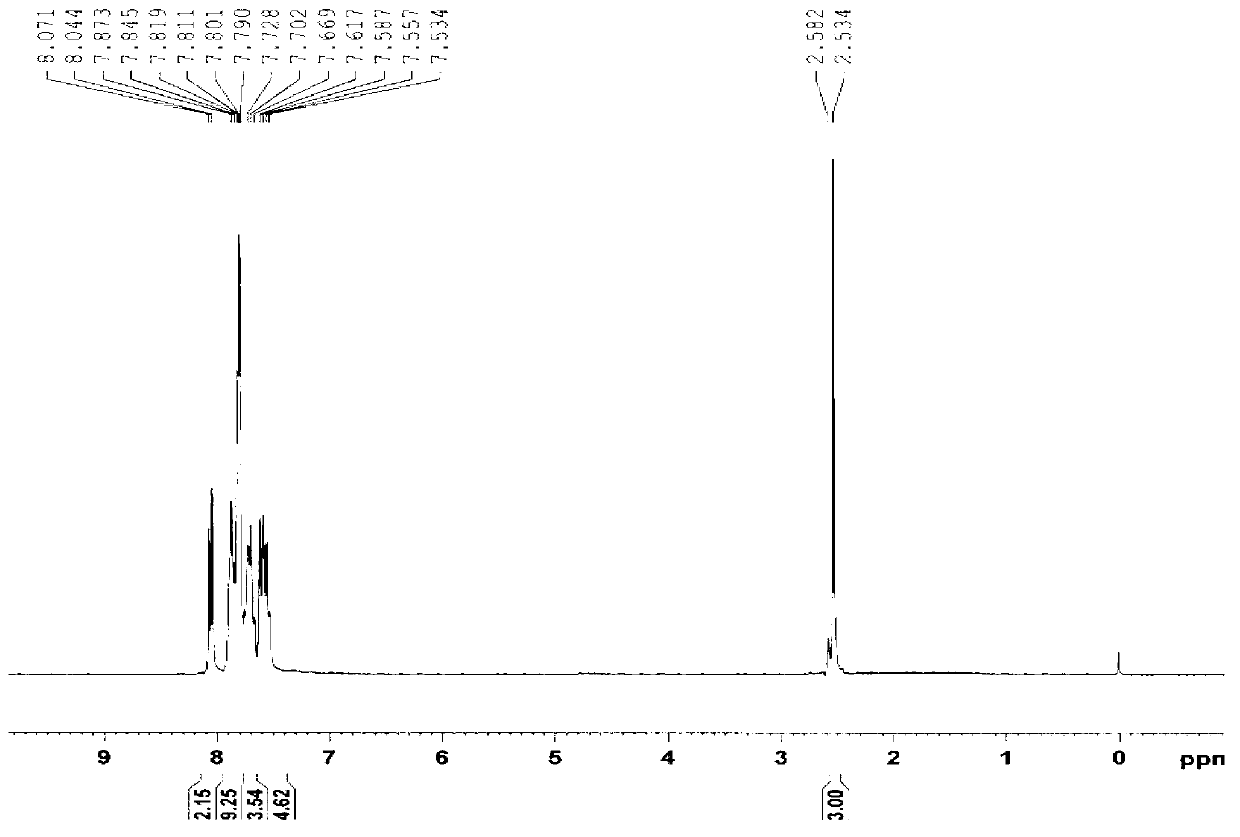
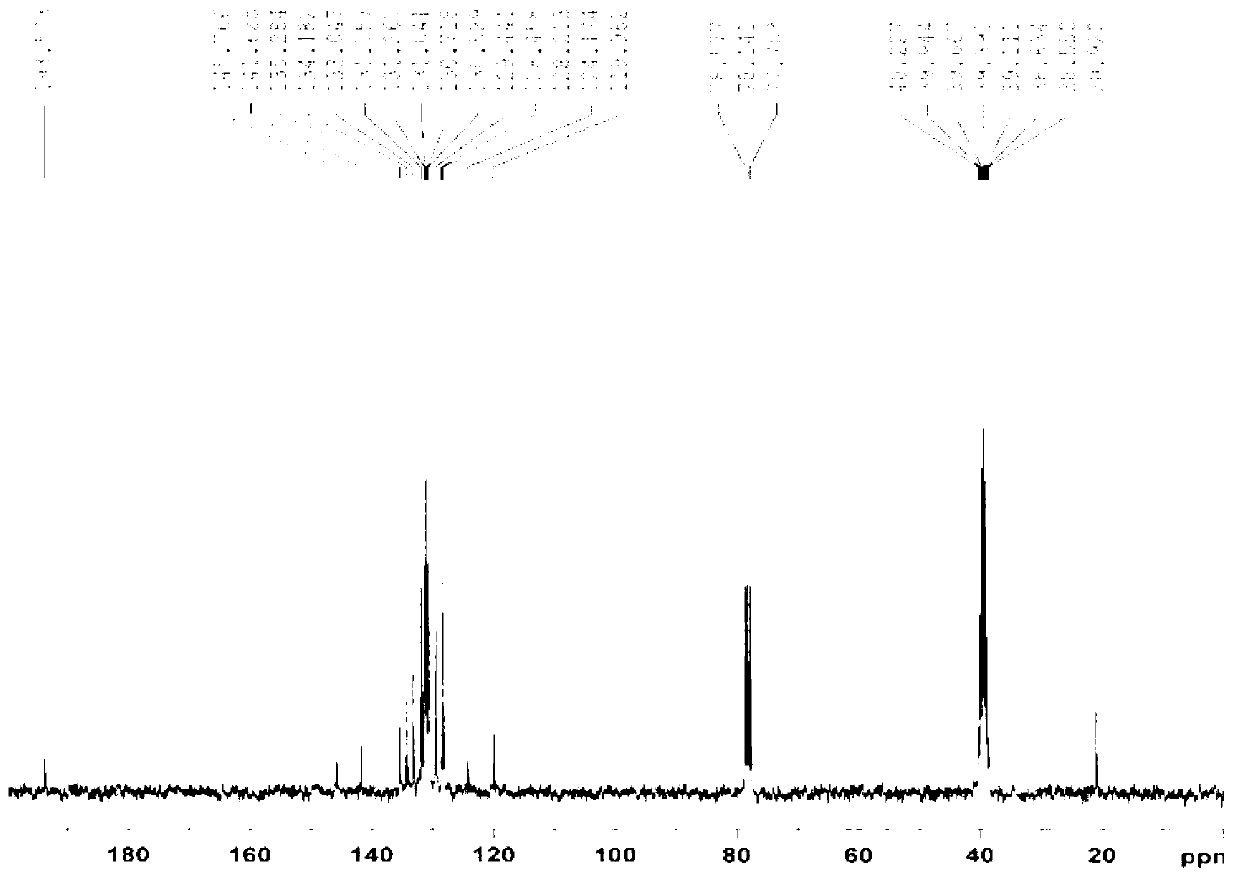
[0023] Product (I): light yellow viscous substance, yield 83.9%. UV spectrum see figure 1 ; H NMR spectrum ( figure 2 ): 1 HNMR (CDCl 3 , 300MHz) δ: 2.53-2.58 (d, CH 3 NMR carbon Spectrum ( image 3 ): 13 C NMR (CDCl 3 , 75MHz) δ: 20.92, 119.98, 124.17, 128.21, 128.46, 129.44, 130.53, 130.72, 131.04, 131.27, 131.76, 133.05, 134.18, 135.28, 141.63, 145.77, 193.57.
Embodiment 2
[0025] Put a magnetic stirring bar, 4-benzoyl-4′-methyl-diphenyl sulfide (BMS) (1.0 mmol), two-(4-tolyl) iodine tri Add fluoromethanesulfonate (1.2mmol) and copper acetate (0.1mmol) into the reactor, mix well, and react in an oil bath at 130°C for 30min under solvent-free conditions. After cooling to room temperature, the mixture was sequentially separated by column chromatography using dichloromethane, ethyl acetate and ethanol as eluents to obtain the product (II) as a milky white solid.
[0026]
[0027] Product (II): milky white solid, yield 91.7%. UV spectrum see Figure 4 ; H NMR spectrum ( Figure 5 ): 1 HNMR (CDCl 3 , 300MHz) δ: 2.44-2.50(s, CH 3 ,6H),7.56-7.63(m,ArH,6H),7.70-7.72(m,ArH,1H),7.78-7.80(m,ArH,6H),7.88-7.91(m,ArH,2H),8.00- 8.03(m,ArH,2H).Carbon NMR ( Figure 6 ): 13 C NMR (CDCl 3 , 75MHz) δ: 20.05, 120.46, 127.84, 128.69, 128.95, 129.88, 130.49, 131.00, 132.68, 134.77, 140.57, 144.65, 193.51.
Embodiment 3
[0029] Put a magnetic stirring bar in a two-necked flask equipped with a condenser, 4-benzoyl-4'-methyl-diphenyl sulfide (BMS) 1.0mmol), di-(4-methylphenyl) iodine trifluoro Methanesulfonate (1.2mmol) and copper acetate (0.1mmol) were added into the reactor, mixed evenly, and reacted in an oil bath at 110°C for 30min under solvent-free conditions. After cooling to room temperature, the mixture was sequentially separated by column chromatography using dichloromethane, ethyl acetate, and ethanol as eluents to obtain the product (II) as a milky white solid with a yield of 54.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com