Novel paliperidone progressively-increased release osmotic pump preparation and preparation method thereof
A controlled-release technology of paliperidone and osmotic pumps, which is applied to pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. To avoid problems such as obvious reaction, to avoid large fluctuations in blood drug concentration, to increase blood drug concentration steadily, and to reduce adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
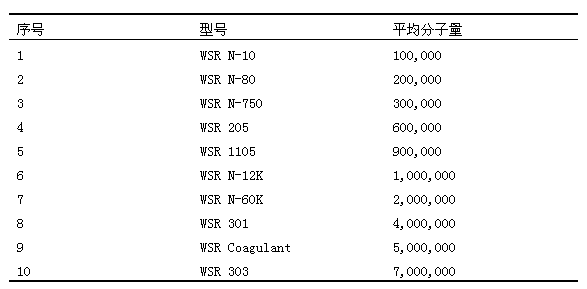
Method used
Image
Examples
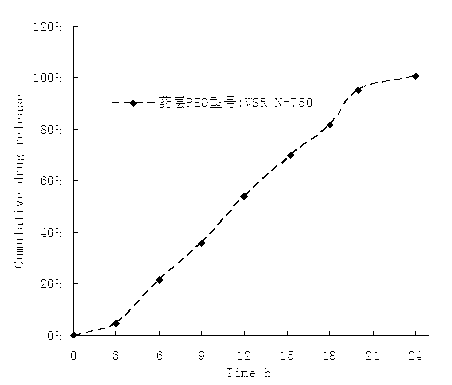
Embodiment 1
[0054]
[0055] Preparation Process:
[0056] (1) Sieve the raw and auxiliary materials of the drug layer, weigh the above substances according to the prescription amount, and mix them evenly;
[0057] (2) Sieve, weigh and mix the auxiliary materials of the booster layer;
[0058] (3) Use a double-layer tablet press machine to laminate the drug layer and booster into a double-layer tablet core;
[0059] (4) Weigh cellulose acetate and porogen according to the prescription amount, dissolve them in acetone and distilled water respectively, mix them evenly to form a coating solution, put the tablet core in the coating pot and spray coat until the weight increases 11%, then dried to remove residual solvent;
[0060] (5) Two holes with a diameter of 1.5 mm are drilled on the side of the drug-containing layer of the coated tablet with a laser or a mechanical drill to obtain paliperidone osmotic pump incremental controlled-release tablets.
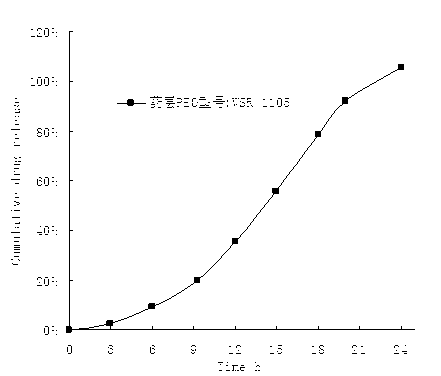
Embodiment 2
[0062]
[0063] Preparation process: (same as Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com