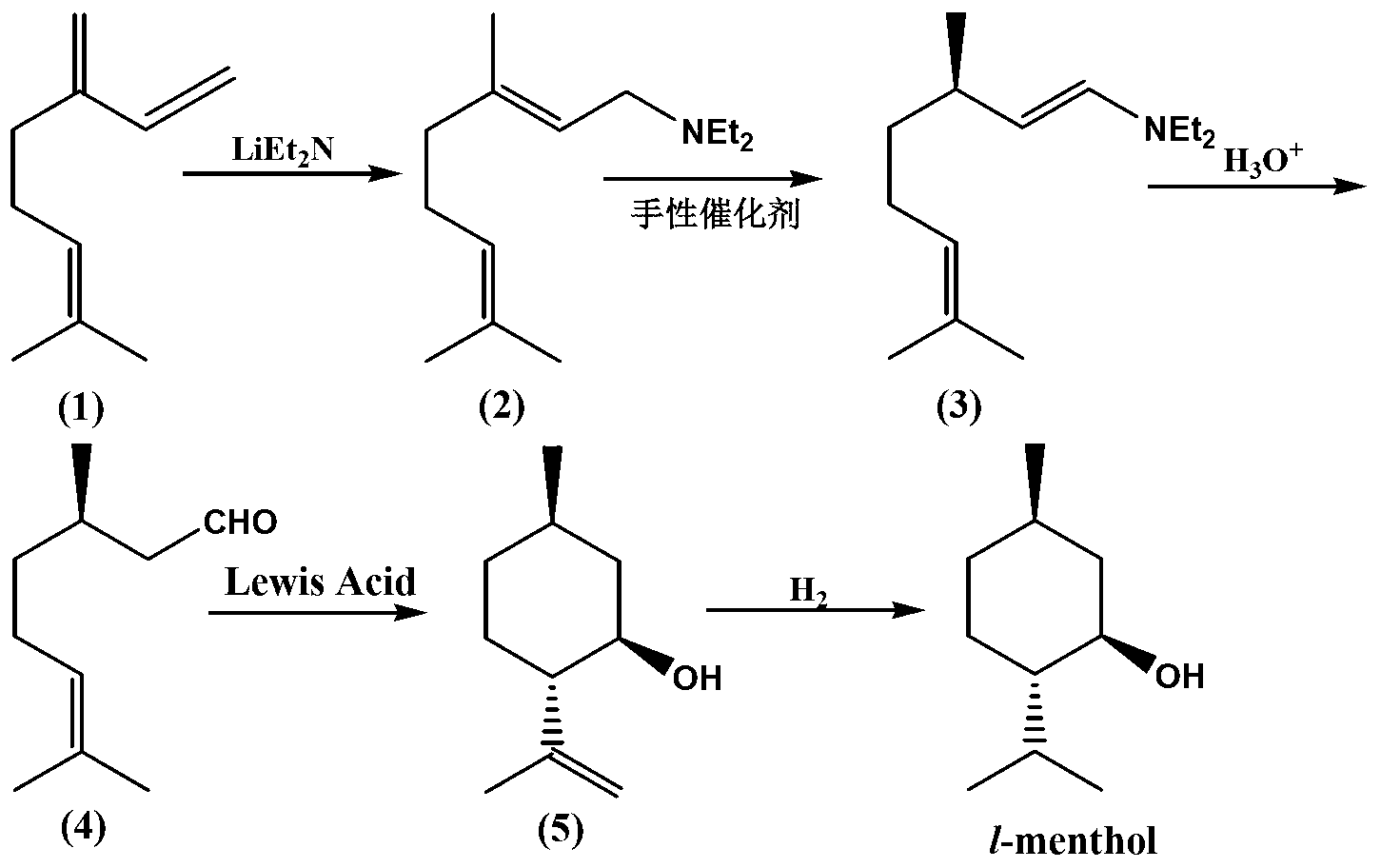
Method for preparing L-menthol intermediate d-citronellal
An intermediate, citronellal technology, which is applied in the field of medicine, can solve problems such as high requirements for operation and equipment, cumbersome recycling process, etc., and achieve the effect of avoiding difficulty in catalyst recovery, reducing production costs, and simple catalyst recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Chiral catalyst preparation:
[0044]Under nitrogen protection, [Rh(COD)Cl] 2 (500mg, 1mmol) was dissolved in 20ml of dry tetrahydrofuran, 1,5-cyclooctadiene COD (0.54ml, 4mmol) was added, and the reaction solution was stirred for 5 minutes, then AgClO 4 (420mg), react at room temperature for 1 hour;
[0045] Under the protection of nitrogen, add PS-BINAP (2.58g) to the above reaction solution, replace nitrogen with hydrogen, keep 3atm hydrogen pressure, stir at room temperature, and detect the reaction by thin layer chromatography until PS-BINAP no longer decreases, about 12 Hour. Stop the reaction, slowly add dry toluene, and then place the reaction bottle in a 0°C refrigerator for crystallization. After removing the solvent, wash with a small amount of toluene, and dry in vacuo to obtain 2.15 g of red crystals, which is the chiral catalyst, with a yield of 80%.
[0046] The chemical structural formula is as follows:
[0047] [Rh(PS-Binap) 2 ] + [ClO 4 ] -
...
Embodiment 2
[0052] Embodiment 2 catalyst preparation
[0053] Under nitrogen protection, [Rh(COD)Cl] 2 (500mg, 1mmol) was dissolved in 20ml of dry dichloromethane, and 1,5-cyclooctadiene COD, (0.54ml, 4mmol) was added. After the reaction solution was stirred for 5 minutes, AgBF was added 4 (430mg), reflux reaction for 1 hour, the above reaction solution was added to PS-BINAP (2.58g), nitrogen was replaced with hydrogen, kept under 1atm hydrogen pressure, stirred at room temperature, TLC detection reaction, until PS-BINAP no longer reduced, After about 5 hours, stop the reaction, slowly add dry ether, and then place the reaction bottle in a 0°C refrigerator for crystallization.
[0054] After the solvent was removed, it was washed with a small amount of toluene and dried in vacuo to obtain 2.20 g of red crystals with a yield of 82%.
[0055] The chemical structural formula is as follows:
[0056] [Rh(PS-Binap) 2 ] + [BF 4 ] -
[0057] Characterized by melting point determination, ...
Embodiment 3
[0061] The preparation of embodiment 3 geranylamine
[0062]
[0063] Add the catalyst of Example 1 into a dry 100 mL reactor with sealed tubes, and replace the system with dry high-purity nitrogen for 3 times. Then add tetrahydrofuran and geranylamine, seal the system, heat to 100°C, and continue the insulation reaction for 15 hours. After the reaction, the system is cooled to room temperature, and then the reaction solution is recovered from the solvent under a reduced pressure of 400 Torr, and then filtered to remove the catalyst. The liquid was directly used in the next reaction without purification. The catalyst was recovered by filtration, washed and weighed dry, with a loss of 0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com