Methods for treating hyperuricemia and related diseases
一种高尿酸血症、血清尿酸的技术,应用在泌尿系统疾病、骨骼疾病、医药配方等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
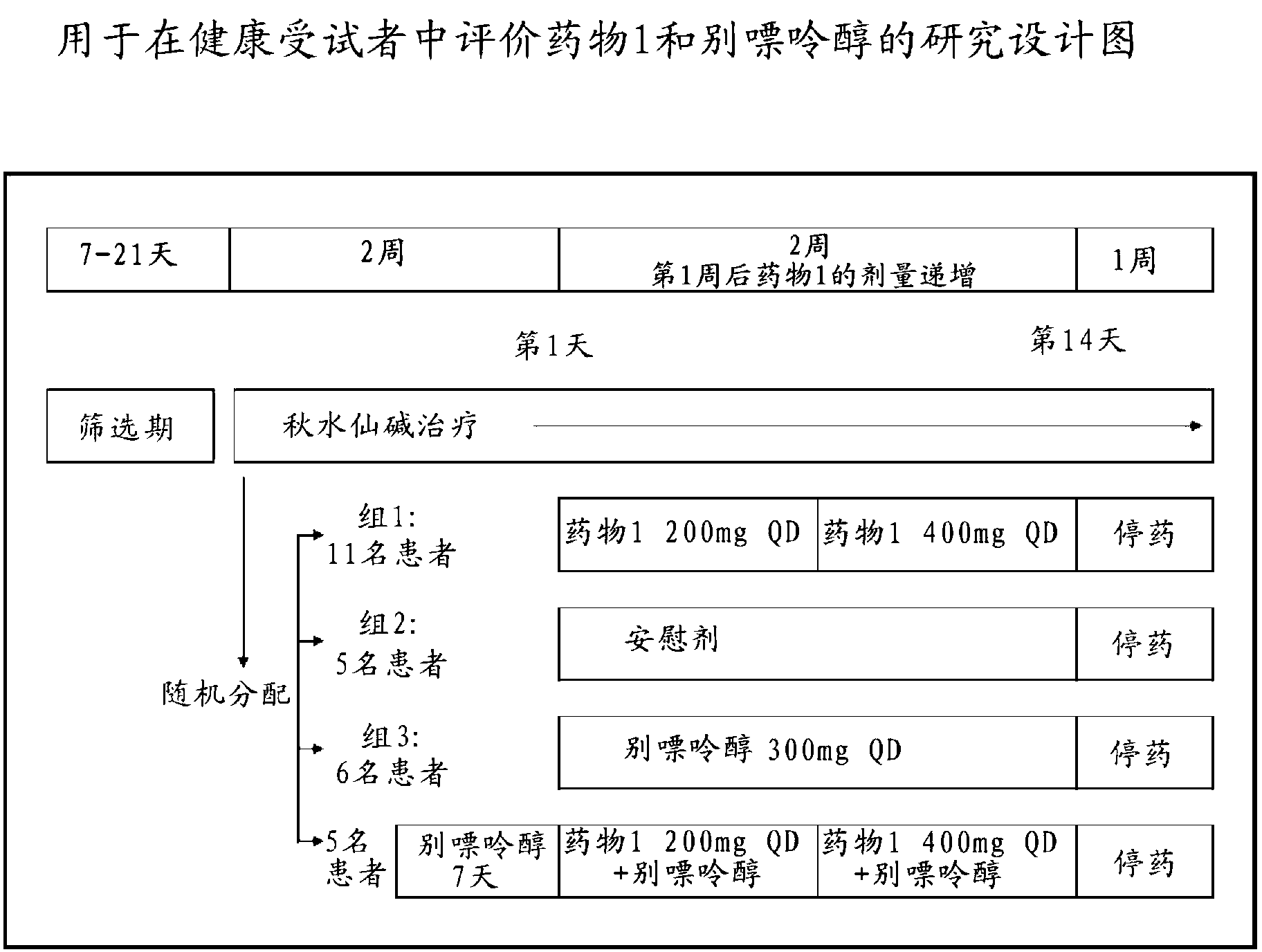
[0167] Research purposes
[0168] • Compare the proportion of subjects with sUA levels <6.0 mg / dL after 2 weeks of continuous treatment with drug 1 versus allopurinol and placebo.
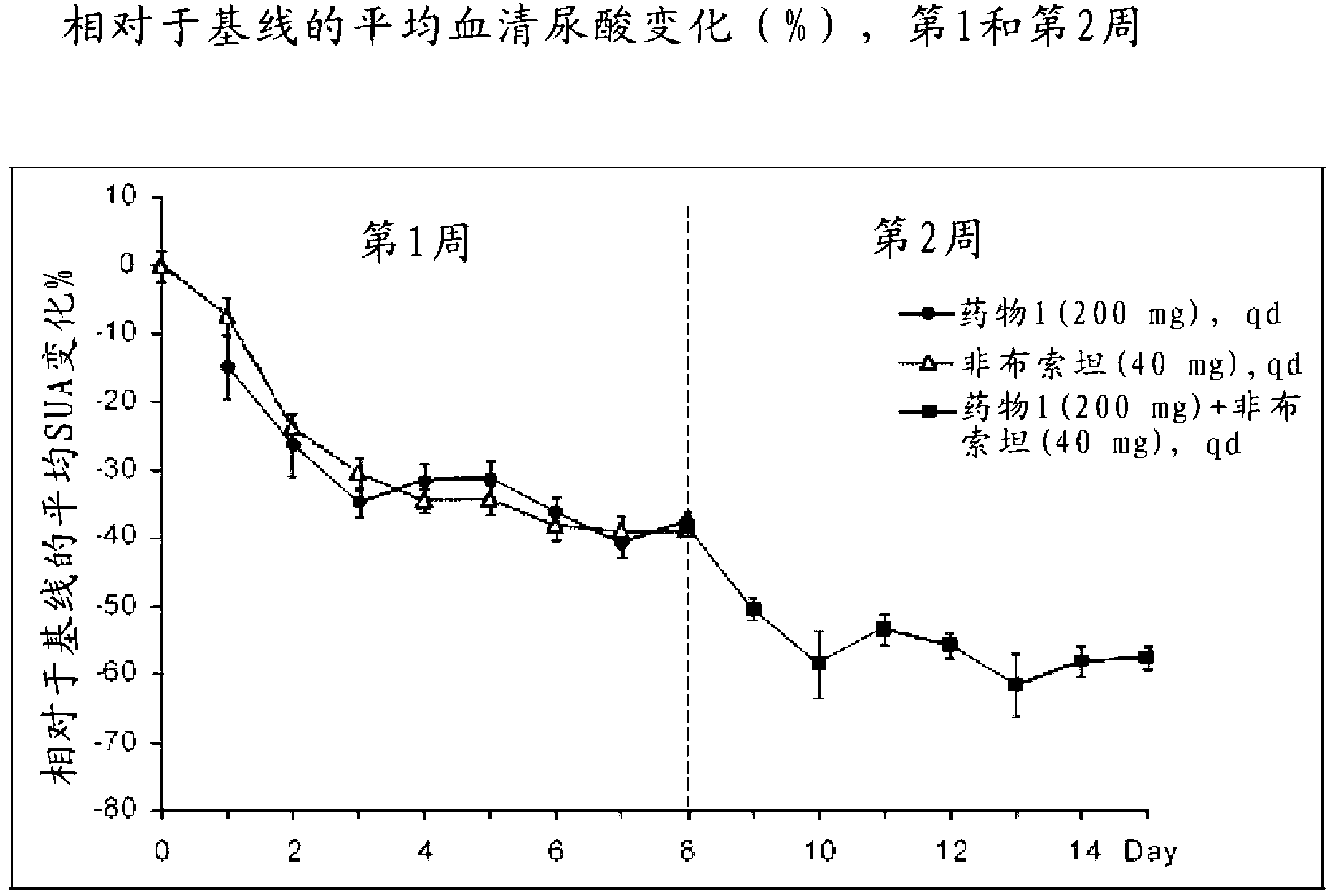
[0169] • To evaluate the percent reduction from baseline in sUA levels after 2 weeks of continuous treatment with Drug 1 in combination with allopurinol.
[0170] • Evaluate the proportion of subjects with sUA levels <6.0 mg / dL, <5.0 mg / dL, and <4.0 mg / dL at each visit.
[0171] ●Evaluate the absolute and percent reduction in sUA levels from baseline at each visit.
[0172] • Evaluate the maximum percent reduction in sUA levels from baseline during the entire treatment period.
[0173] • Evaluate the percent change in 24-hour urine uric acid levels from baseline to Day 15.
[0174] • To evaluate the safety and tolerability of Drug 1 in subjects with gout.
[0175] • To evaluate the pharmacokinetics, safety and tolerability of Drug 1 in combination with allopurinol in subjects with gout.
[017...
Embodiment 2
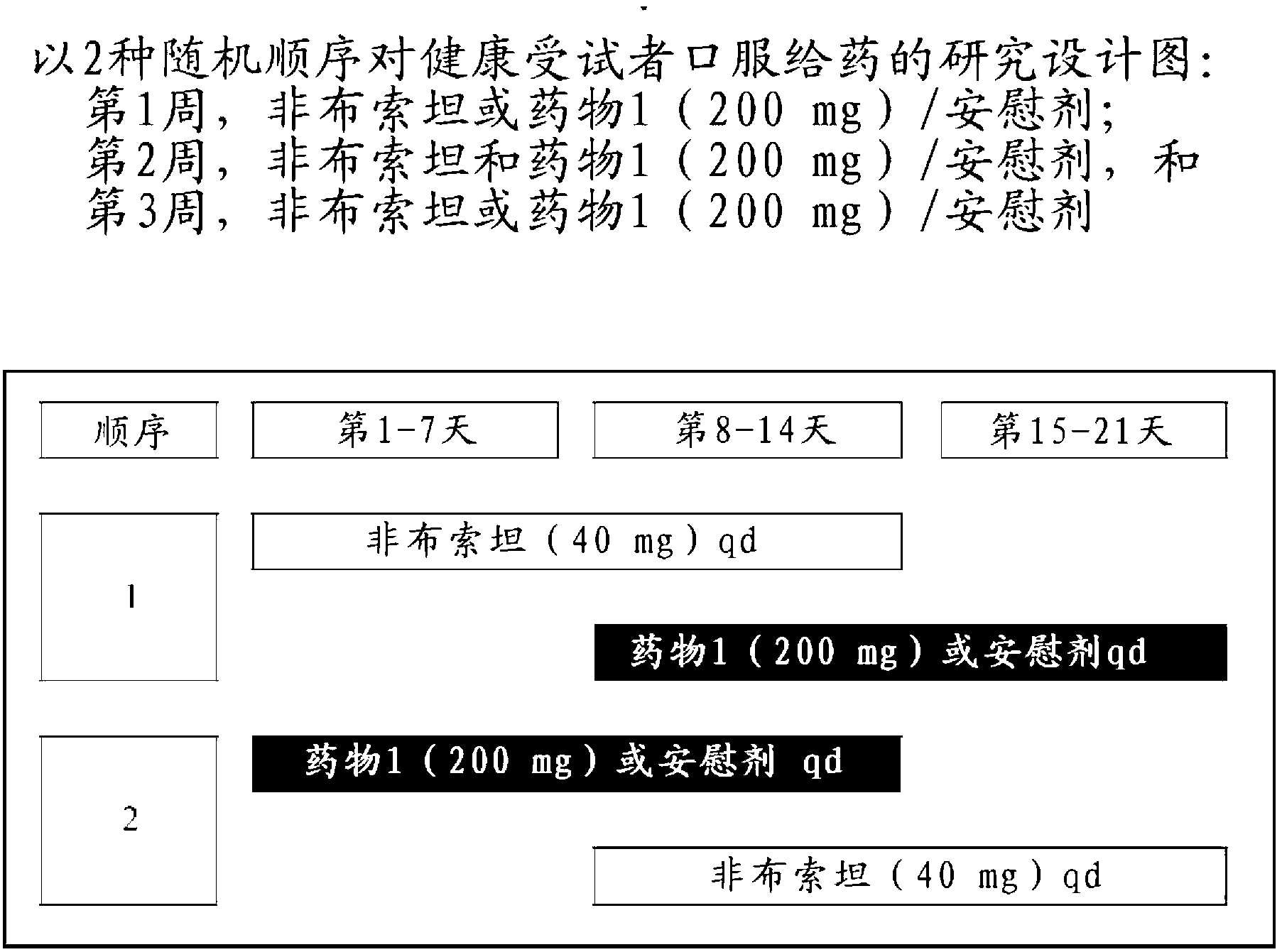
[0357] Drug 1 was tested according to the clinical trial protocol described in Example 1.
[0358] The actual selection is as follows:
[0359] Cohort 1: 21 subjects - 11 randomly assigned to drug 1 group
[0360] -5 randomized to placebo group
[0361] -5 randomized to open-label allopurinol group
[0362] Group 2: 6 subjects - 5 randomly assigned to drug 1 + allopurinol group
[0363] -1 assigned to placebo + allopurinol group
[0364] Preliminary Safety Summary (Group 1 only)
[0365] Drug 1 was well tolerated in this study, with no SAEs, deaths, or discontinuations due to adverse events, and no clinically significant changes in physical examination results or vital signs. There were no clinically significant ECG findings (including interval measurements), and no increase in dose-related adverse events (all events were transient and mild to moderate in severity).
[0366] Two patients had a >30% increase in serum creatinine (SCr) when taking 400 mg QD (grade 1 AE), no...
Embodiment 3
[0372] According to the clinical trial protocol described in Example 1, BCX4208 (as 7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-1-yl)methyl)-3H-pyrrole [3,2-d]pyrimidin-4(5H)-one) was used instead of allopurinol to evaluate drug 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com