Preparation method of metal organic framework material MIL-100 (Fe)
A metal-organic framework and organic ligand technology, applied in the direction of iron-organic compounds, can solve the problems of harsh synthesis conditions, low product yield, difficult industrial production, etc., achieve high quality, overcome low yield and reduce synthesis energy. consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 2.02g Fe(NO 3 ) 3 .9H 2 O. Add 0.70g of 1,3,5-benzenetricarboxylic acid into a three-necked flask filled with 5mL of deionized water, stir it magnetically for about 30 minutes, reflux and condense, raise the temperature to 95°C and keep it at a constant temperature for 12 hours. The selected reactor is a three-necked flask Add a reflux condenser. After the reaction, the sample was filtered, washed with a sufficient amount of deionized water, and placed in a drying oven for constant temperature drying for 5 hours.
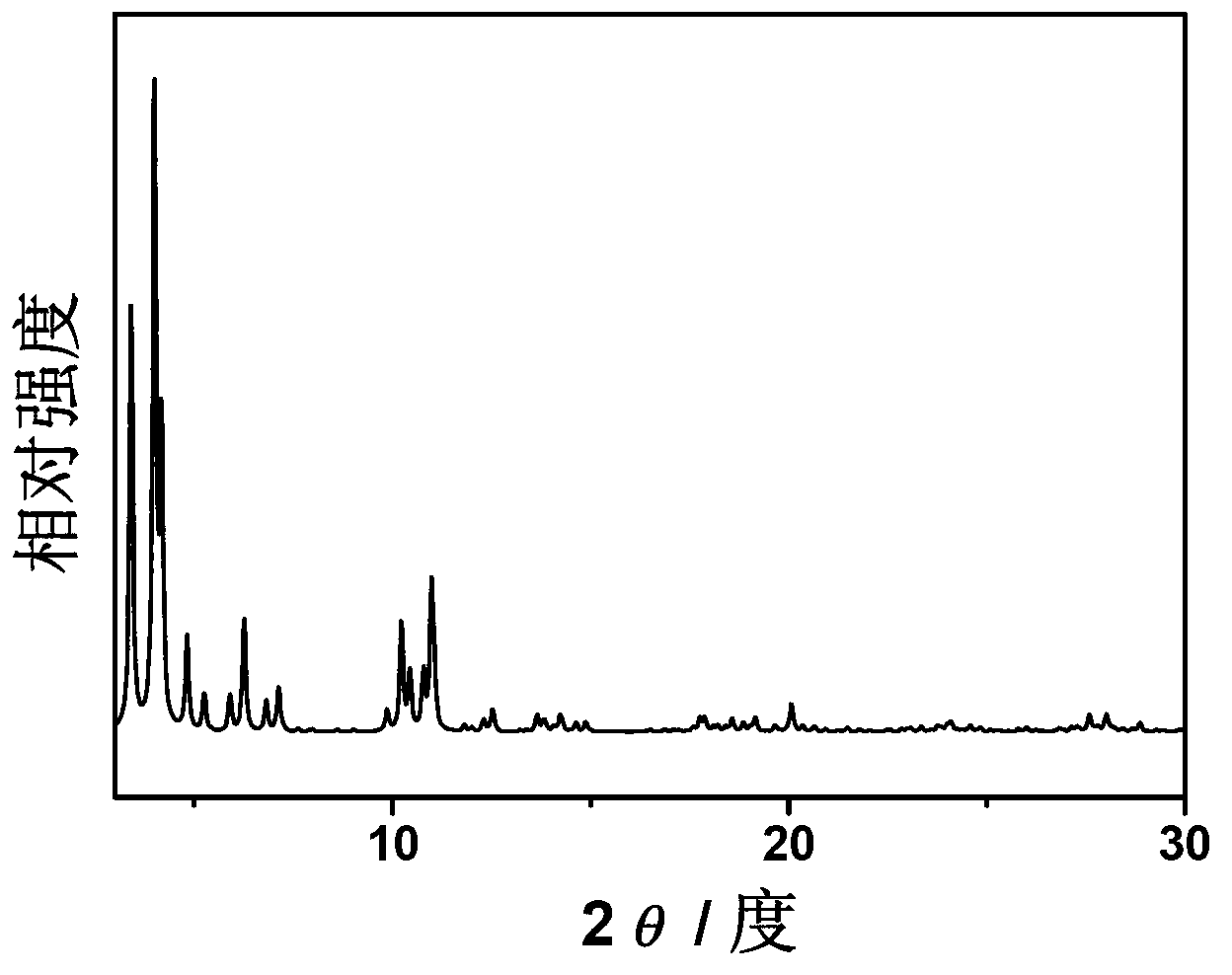
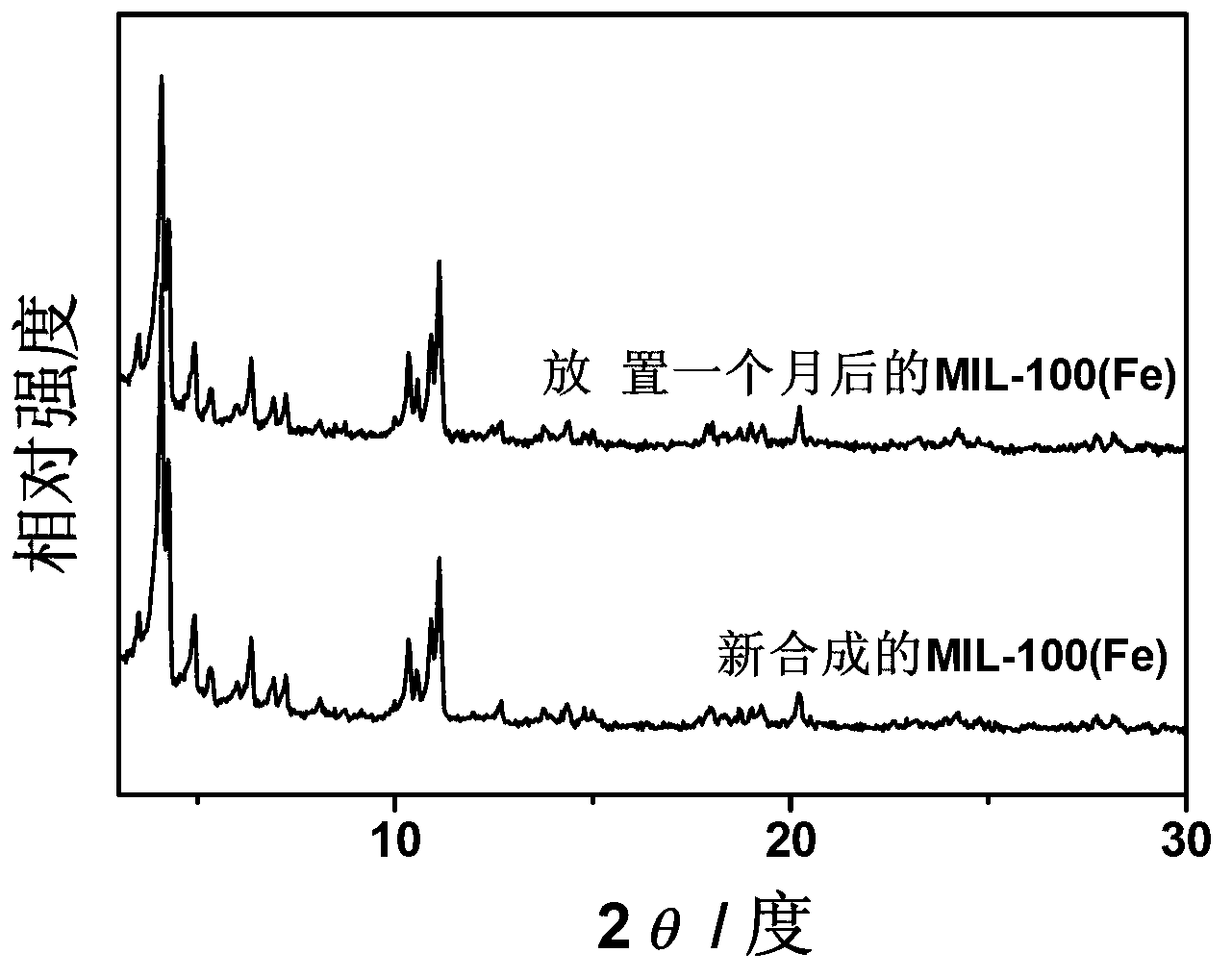
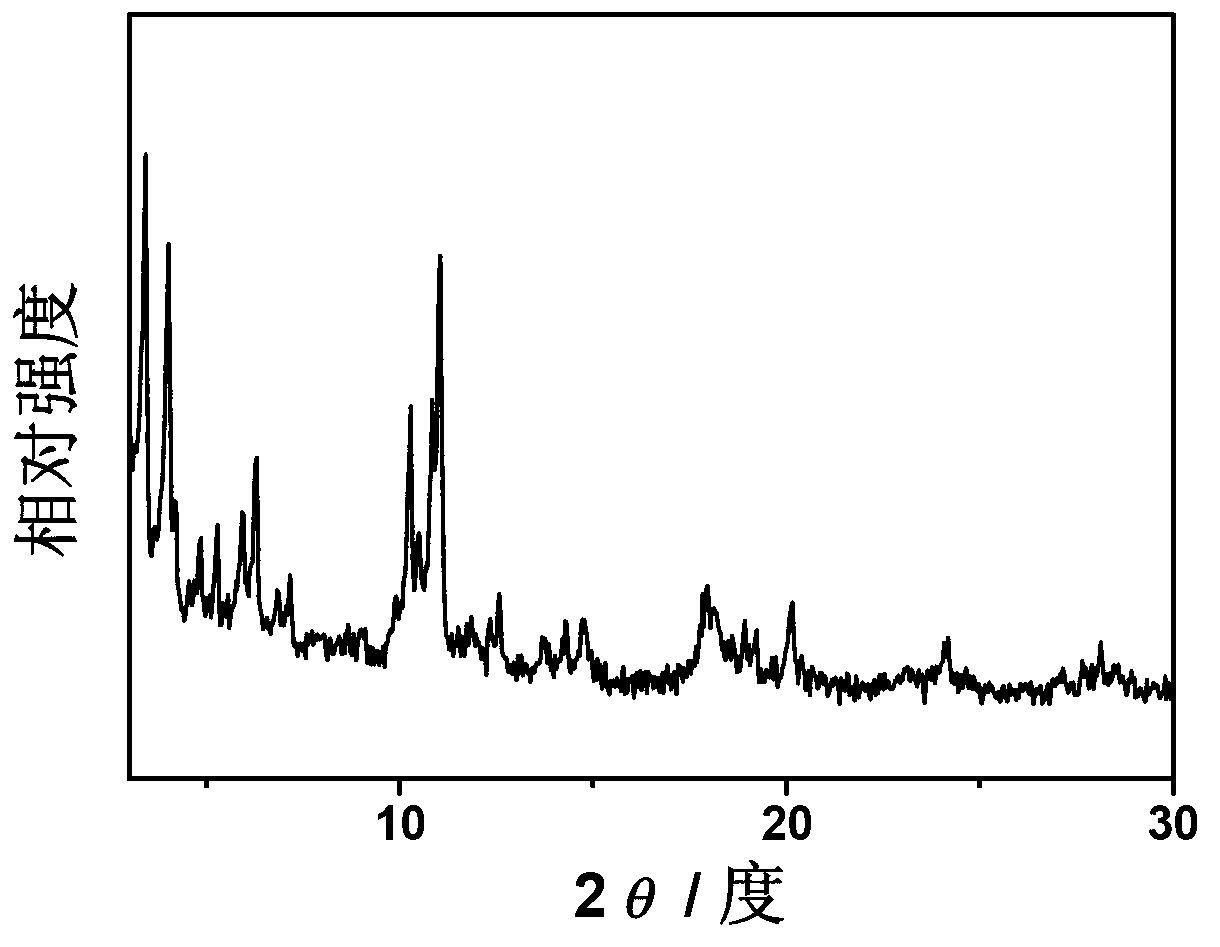
[0021] The dried samples were further purified with ethanol and ammonium fluoride, respectively. First, treat with absolute ethanol at 70°C for 10h, then treat with 30mmol / L ammonium fluoride solution at 80°C for 10h, and finally wash fully with deionized water. The drying temperature was 150°C, and the drying time was 10 hours to obtain a light orange powder. Through XRD analysis, its characteristic peak is consistent with the fitted MIL-100 (Fe) charac...
Embodiment 2
[0023] Sequentially add 0.81g FeCl 3 .6H 2 O. Add 0.51g of 1,3,5-trimethylbenzenetricarboxylate into a three-necked flask filled with 15mL of deionized water, stir it magnetically for about 30 minutes, reflux and condense, raise the temperature to 95°C and keep it at a constant temperature for 12 hours. The selected reactor Add a reflux condenser to the three-necked flask. After the reaction, the sample was filtered, washed with a sufficient amount of deionized water, and placed in a drying oven for constant temperature drying for 5 hours.
[0024] The dried samples were further purified with ethanol and ammonium fluoride, respectively. First, treat with absolute ethanol at 60°C for 15h, then treat with 40mmol / L ammonium fluoride solution at 70°C for 15h, and finally wash fully with deionized water. The drying temperature was 100°C, and the drying time was 10 hours to obtain a light orange powder. Through XRD analysis, its characteristic peak is consistent with the fitted ...
Embodiment 3
[0026] 2.02g Fe(NO 3 ) 3 .9H 2 O. Add 0.63g of 1,3,5-trimethylbenzenetricarboxylate into a three-necked flask filled with 5mL of deionized water, stir magnetically for about 30min, reflux and condense, heat up to 80°C and keep at constant temperature for 20h, the selected reactor Add a reflux condenser to the three-necked flask. After the reaction, the sample was filtered, washed with a sufficient amount of deionized water, and placed in a drying oven for constant temperature drying for 5 hours.
[0027] The dried samples were further purified with ethanol and ammonium fluoride, respectively. First, treat with absolute ethanol at 80°C for 20h, then treat with 50mmol / L ammonium fluoride solution at 60°C for 10h, and finally wash fully with deionized water. The drying temperature was 150°C, and the drying time was 5 hours to obtain a light orange powder. According to XRD analysis, its characteristic peaks are consistent with the fitted MIL-100(Fe) characteristic peaks, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com