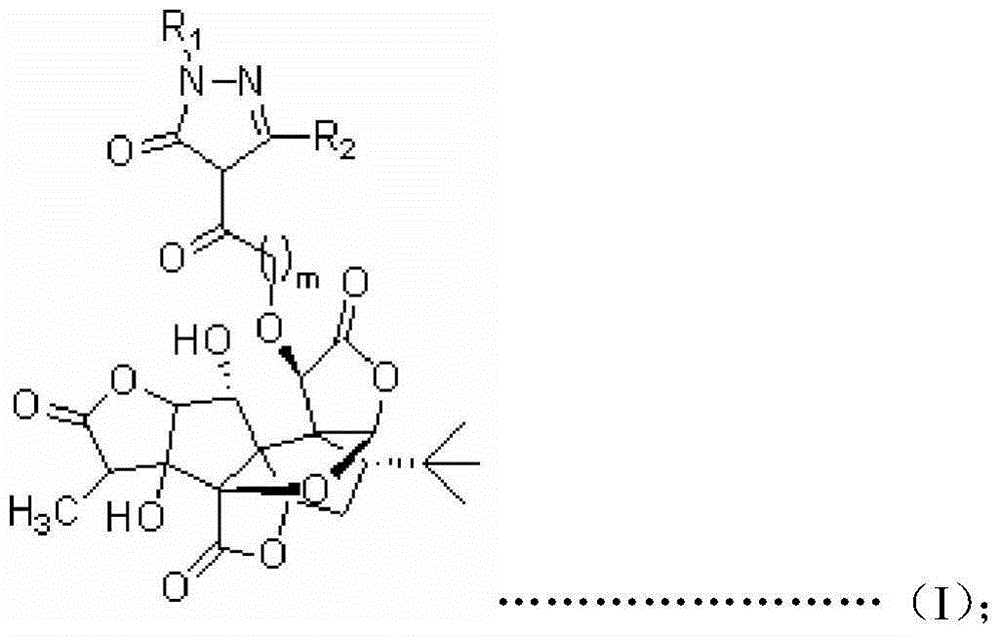
Ginkgolide B derivative based on double target spots of PAF (Platelet Activating Factor)/ROS (Reactive Oxygen Species), and preparation method of derivative
A technology of ginkgolide and derivatives, applied in the directions of drug combination, antidote, blood diseases, etc., to achieve the effect of excellent inhibition of lipid peroxidation, avoid drug quality control difficulties, and inhibit oxidative stress damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
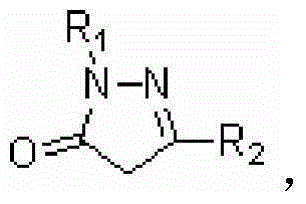
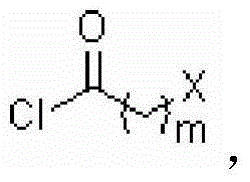
[0054] Example 1: Synthesis of Intermediate A - 1-phenyl-3-methyl-4-(2`-bromoacetyl)-5-pyrazolone
[0055]
[0056] Weigh 4.5 g 1-phenyl-3-methyl-5-pyrazolone and 3.8 g Ca(OH) 2 In the reaction flask, add 70 ml of 1,4-dioxane as the reaction medium. Then 2 ml of bromoacetyl chloride was added dropwise to the reaction system, the drop was completed within 15 min, and the reaction was heated under reflux for 1 h. After the reaction, filter to remove insoluble Ca(OH) 2 , and add 30 ml of 1 mol / L hydrochloric acid aqueous solution to the reaction system, collect the generated crystals, MeOH-H 2 O recrystallized to obtain product 4.95g, yield 65%.
[0057] 1 H NMR (CDCl 3 , 500MHz): 7.58-7.64 (m, 2H), 7.18-7.26 (m, 2H), 6.98-7.02 (m, 1H), 4.32(s, 2H), 3.27 (s, 1H), 0.87 (s, 3H ).
Embodiment 2
[0058] Example 2: Synthesis of Intermediate A—1-methyl-3-trifluoromethyl-4-(4`-bromobutyryl)-5-pyrazolone
[0059]
[0060] The synthesis process is as in Example 1, except that 1-phenyl-3-methyl-5-pyrazolone in Example 1 is replaced by 1-methyl-3-trifluoromethyl-5-pyrazoline ketone. Yield 57%.
[0061] 1 H NMR (CDCl 3 , 500MHz): 3.45 (t, 2H), 3.31 (s, 1H), 2.64 (s, 3H), 2.45 (t, 2H), 2.11(m, 2H).
Embodiment 3
[0062] Embodiment 3: the synthesis of target product (I-1)
[0063]
[0064] Target product I-1
[0065] Weigh 0.85g GB and 0.83g anhydrous K 2 CO 3 In the reaction flask, add 30 ml CH 3 CN as the reaction solvent, followed by dropwise addition of 0.885 g of 1-phenyl-3-methyl-4-(2`-bromoacetyl)-5-pyrazolone 3 The CN solution was dropped within 30 min, and the reaction was continued at room temperature for 20 h. After the reaction, remove the insoluble matter by filtration, concentrate the filtrate under reduced pressure, add 20 ml of 2 mol / L hydrochloric acid aqueous solution and wash with CH 2 Cl 2 or CHCl 3 Extracted three times, combined extracts, washed with water, anhydrous MgSO 4 dry. The crude product was concentrated under reduced pressure to obtain a crude product, which was separated and purified by column chromatography, petroleum ether / acetone = 2:1 as the eluent, and 0.918 g of a colorless solid was finally obtained with a yield of 72%.
[0066] 1 H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com