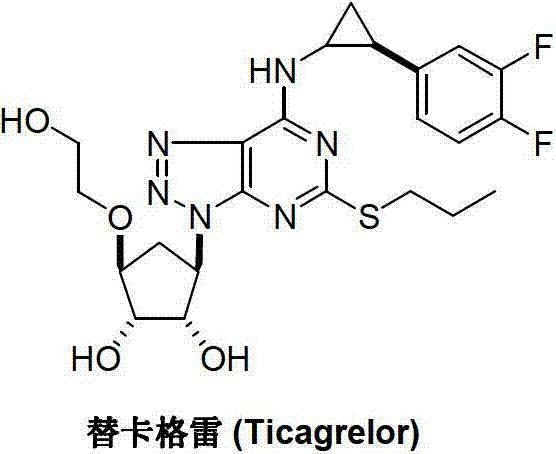
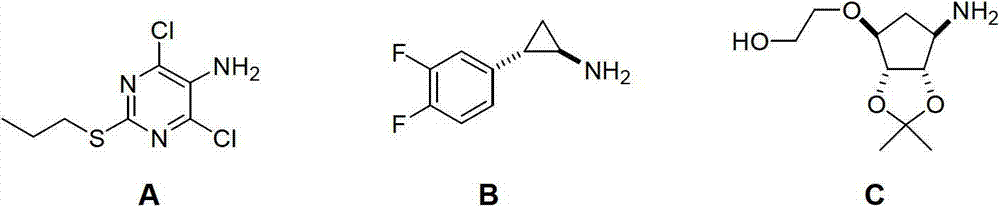
Method for preparing trans-(1R,2S)-2-(3,4-difluorophenyl) cyclopropylamine
A technology of difluorophenyl and cyclopropylamine, which is applied in the field of preparation of ticagrelor intermediate trans--2-cyclopropylamine, can solve the problems of long steps, rare raw materials, heavy pollution, etc., and is suitable for large-scale industrialization The production and reaction conditions are mild and the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 3,4-difluorobenzaldehyde (I) (7.1g, 0.05mol), nitromethane (7.7g, 0.1mol) and 50mL of ethanol into the reaction flask, start stirring, and add ammonium acetate (3.85g, 0.05mol) , acetic acid (4.2g, 0.07mol) and phase transfer catalyst cetyltrimethylammonium bromide (0.9g, 2.5mmol, 5%eq), warming up to 75-80 ° C, reflux reaction for 5 hours, TLC detection reaction Finish. Concentrate under reduced pressure to 1 / 3 of the volume, add 100 mL of water, cool to 10°C, and stir to crystallize. After filtration and drying, 7.8 g of light yellow solid β-(3,4-difluorobenzene)nitroethylene (II) was obtained, with a yield of 84.3%.
Embodiment 2
[0038] Add trans-1,2-cyclohexanediamine (0.34g, 3mmol), 3,5-tert-butyl salicylaldehyde (0.70g, 3mmol) and 25mL of dioxane in the reaction flask, start stirring, keep The reaction was stirred at 55°C for 1 hour. Diethylzinc (2.5 g, 30 mmol) and diiodomethane (12.1 g, 45 mmol) were added to the reaction flask and stirring was continued for 30 minutes. Dissolve β-(3,4-difluorobenzene)nitroethylene (II) (2.8g, 15mmol) in 10mL of dioxane, drop it into the above reaction system within 30 minutes, and keep it at 55°C for 5 hours. TLC detects that the reaction is complete. Cool to room temperature, add ethyl acetate and water, and separate layers. The organic phase was washed with brine and dried over anhydrous sodium sulfate. The solvent was recovered by distillation under reduced pressure, and the residual oil was recrystallized from n-hexane to obtain 2.6 g of trans-2-(3,4-difluorophenyl)nitrocyclopropane (III) as a light yellow solid, with a yield of 87.0%.
Embodiment 3
[0040] Add trans-2-(3,4-difluorophenyl)nitrocyclopropane(III) (3.0g, 15mmol), sodium dithionite (15.7g, 90mmol), 50mL of toluene and 50mL of water into the reaction flask, At the same time, 0.25 g of tetrabutylammonium bromide was added. Start stirring, keep stirring at 65° C. for 4 hours, and TLC detects that the reaction is complete. Cool to room temperature, separate the organic layer, wash the water layer twice with toluene, combine the organic phases, wash with 5% sodium bicarbonate and water successively, dry, and recover the solvent by distillation under reduced pressure, and dissolve the residual oily matter with ethyl acetate. Add S-mandelic acid (2.28g, 15mmol), stir at room temperature for 6-8 hours, cool down to 0°C, filter, and the obtained solid is S-mandelic acid salt of the target product. The solid was placed in 50 mL of water, the pH was adjusted to 12-13 with 30% sodium hydroxide, extracted three times with ethyl acetate, and the organic phases were combined....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com