Method for preparing loratadine tablet
A technology of loratadine and tablets, which is applied in the field of drug preparation, can solve problems such as the increase of impurities, and achieve the effect of improving the dissolution rate and improving the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
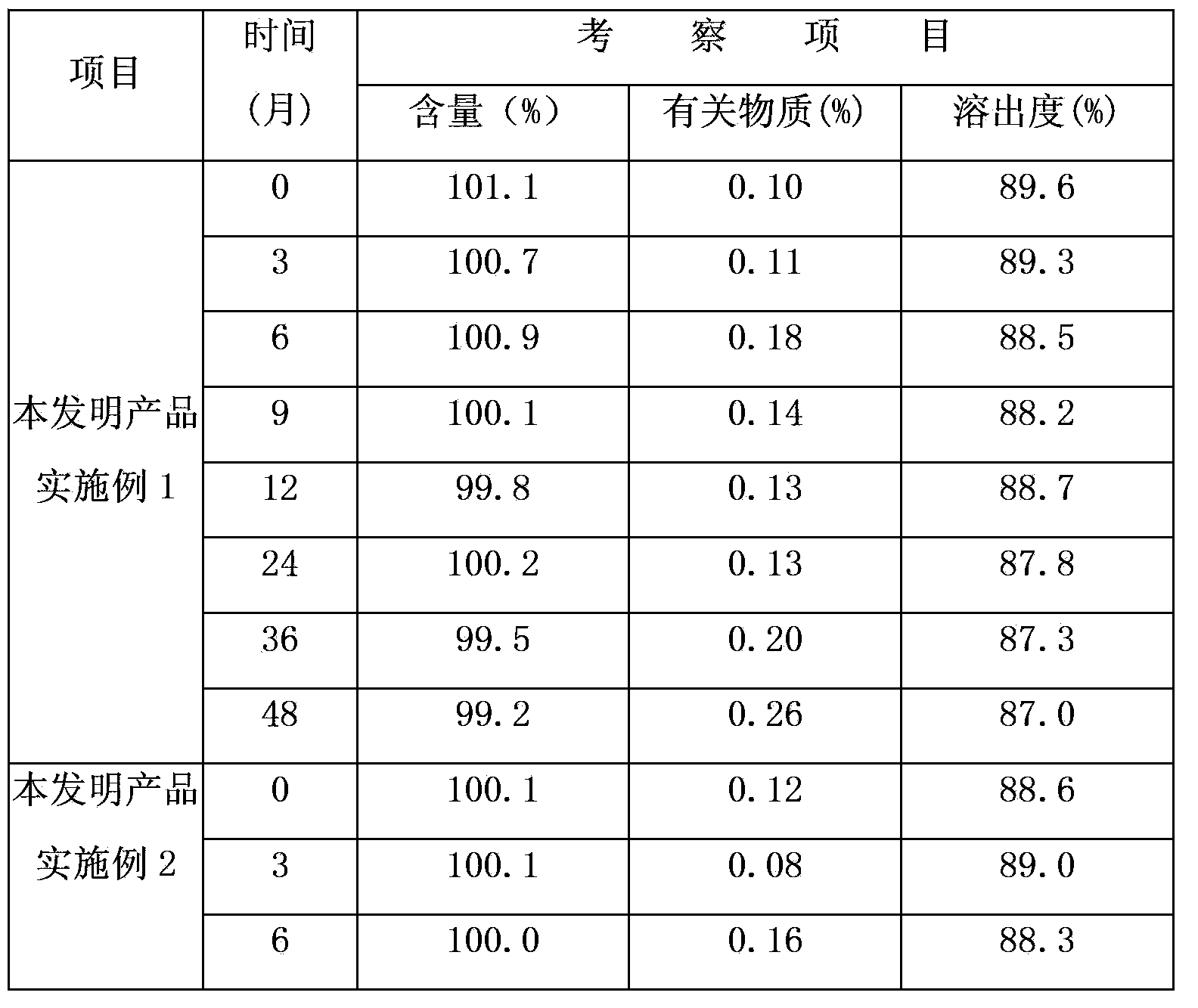
Embodiment 1
[0019] Drug specifications: 10mg
[0020] The formula is as follows:
[0021] Vegetarian tablet formula Proportion (weight percentage) 1000 tablets formula (g) Loratadine 9.52%10.0 Citrate 11.43%12.0 lactose 70.95%74.5 Copovidone 1.91%2.0 Crospovidone 5.24%5.5 Magnesium stearate 0.95%1.0 total 100%105
[0022] A preparation method of loratadine tablets, according to the following steps:
[0023] 1. According to the above-mentioned drug formula, add citric acid to 95% ethanol solution, stir to dissolve, and prepare an inclusion solution. The mass volume ratio of citric acid to 95% ethanol is 1:100;
[0024] 2. Pass loratadine through a 100-120 mesh sieve, put it in a boiling drying bed, spray the inclusion liquid in a boiling state, and dry at 45-55°C to prepare loratadine acidic inclusion compound ;
[0025] 3. Mix the loratadine acid inclusion compound, lactose, copovidone, crospovidone, and magnesium stearate evenly, add them to the hopper of the tablet press, and compress ...
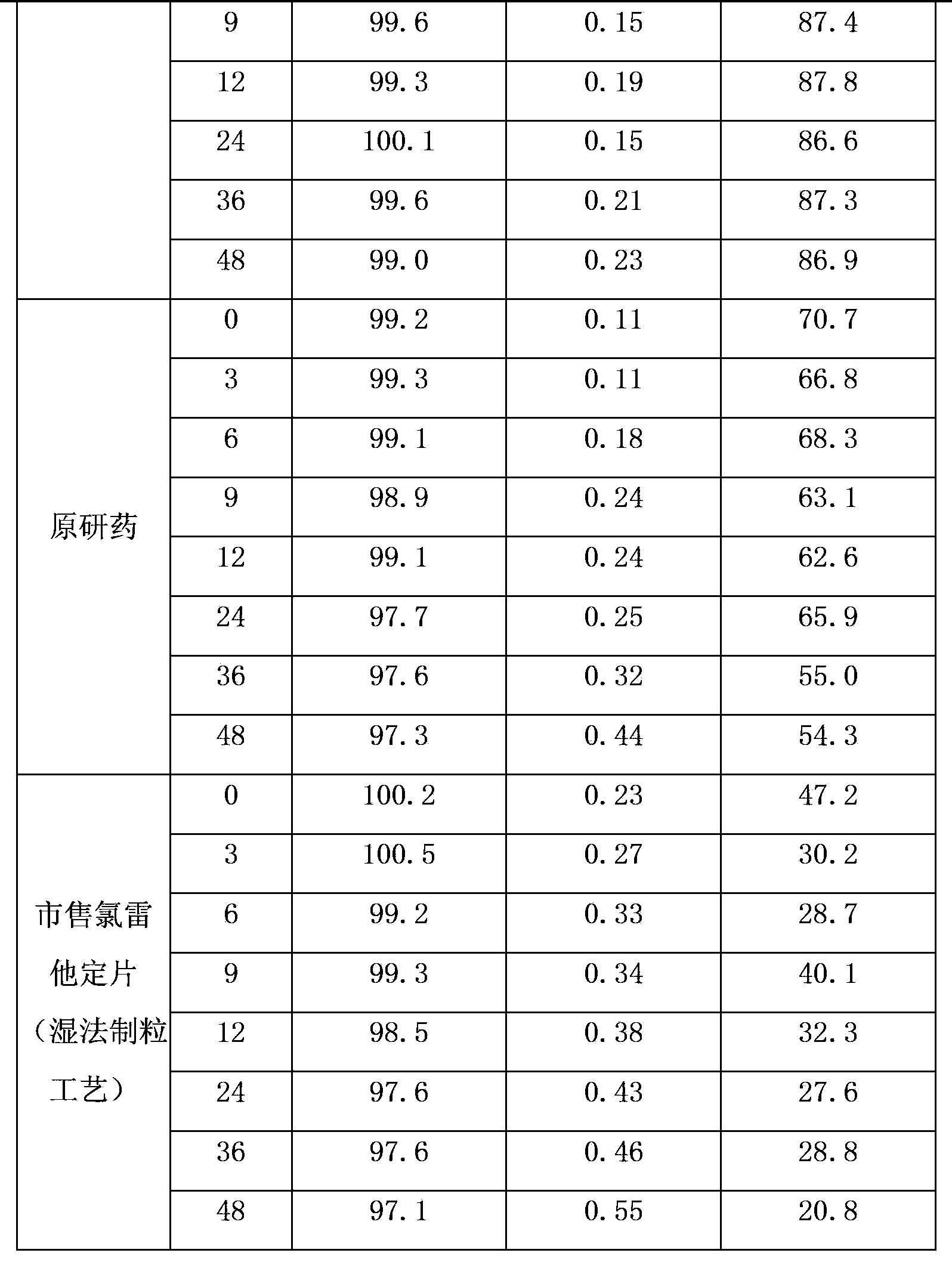
Embodiment 2
[0027] Drug specifications: 5mg
[0028] The formula is as follows:
[0029] Vegetarian tablet formula Proportion (weight percentage) 1000 tablets formula (g) Loratadine 4.76%5.0
[0030] Citrate 5.24%5.5 lactose 81.71%85.8 Copovidone 2.10%2.2 Crospovidone 5.52%5.8 Magnesium stearate 0.67% 0.7 total 100%105
[0031] A preparation method of loratadine tablets, according to the following steps:
[0032] 1. According to the above drug formula, add citric acid to 95% ethanol solution, stir to dissolve, and prepare an inclusion solution. The mass volume ratio of citric acid to 95% ethanol is 0.5:100;
[0033] 2. Pass loratadine through a 100-120 mesh sieve, put it in a boiling drying bed, spray the inclusion solution in a boiling state, and dry at 45-55°C to prepare loratadine acidic inclusion compound ;
[0034] 3. Mix the loratadine acid inclusion compound, lactose, copovidone, crospovidone, and magnesium stearate evenly, add them to the hopper of the tablet press, and compre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com