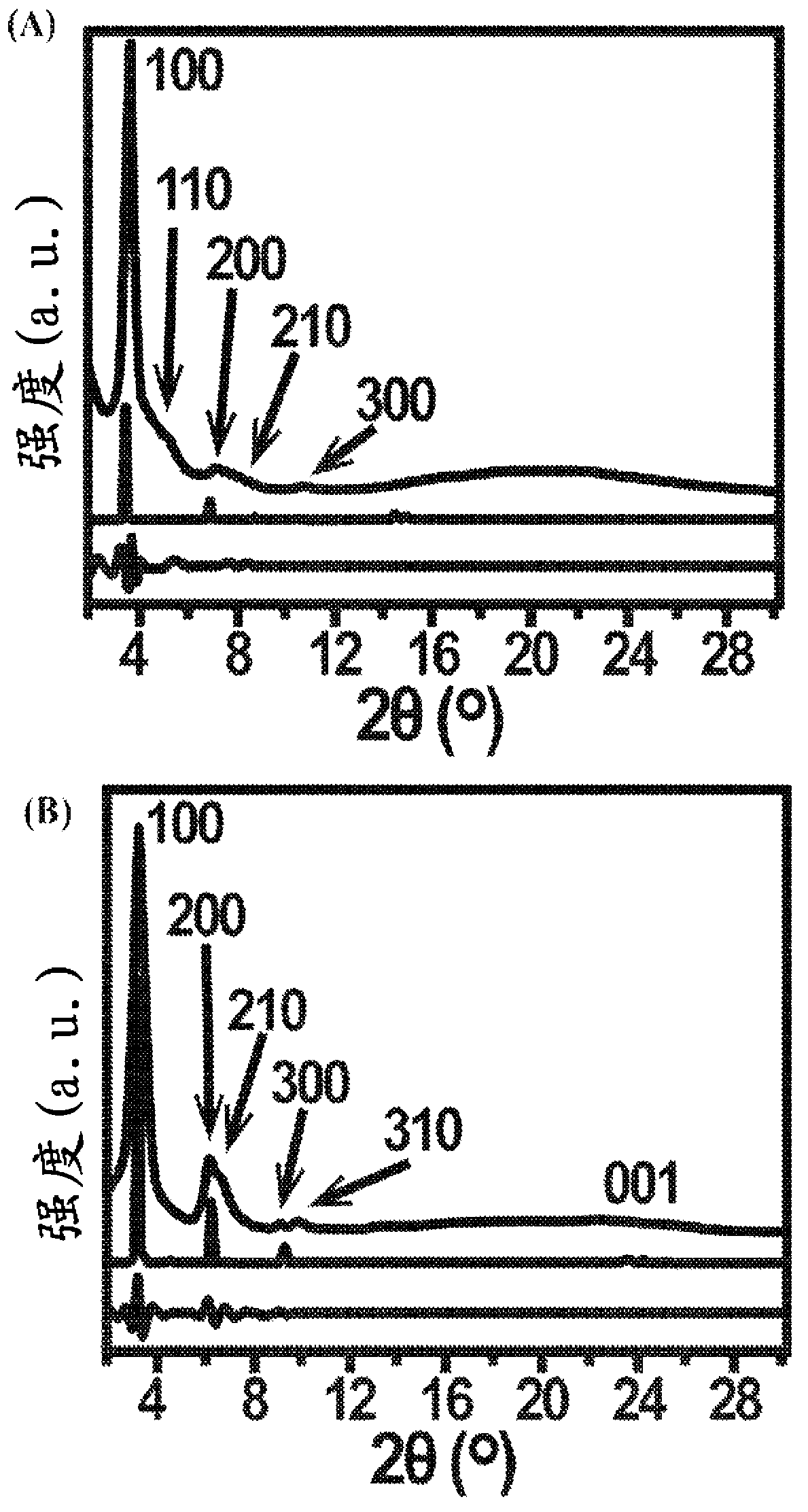
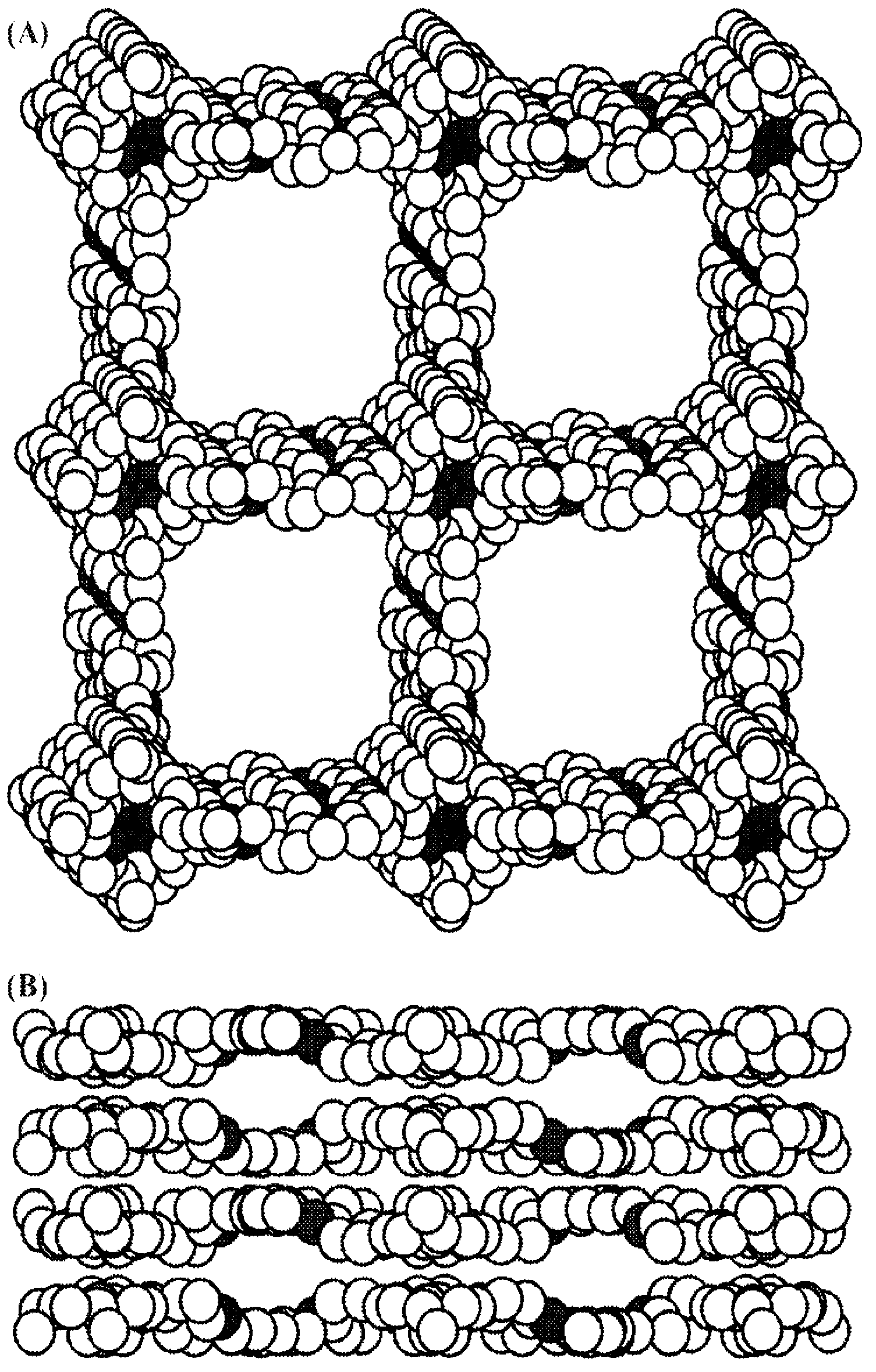
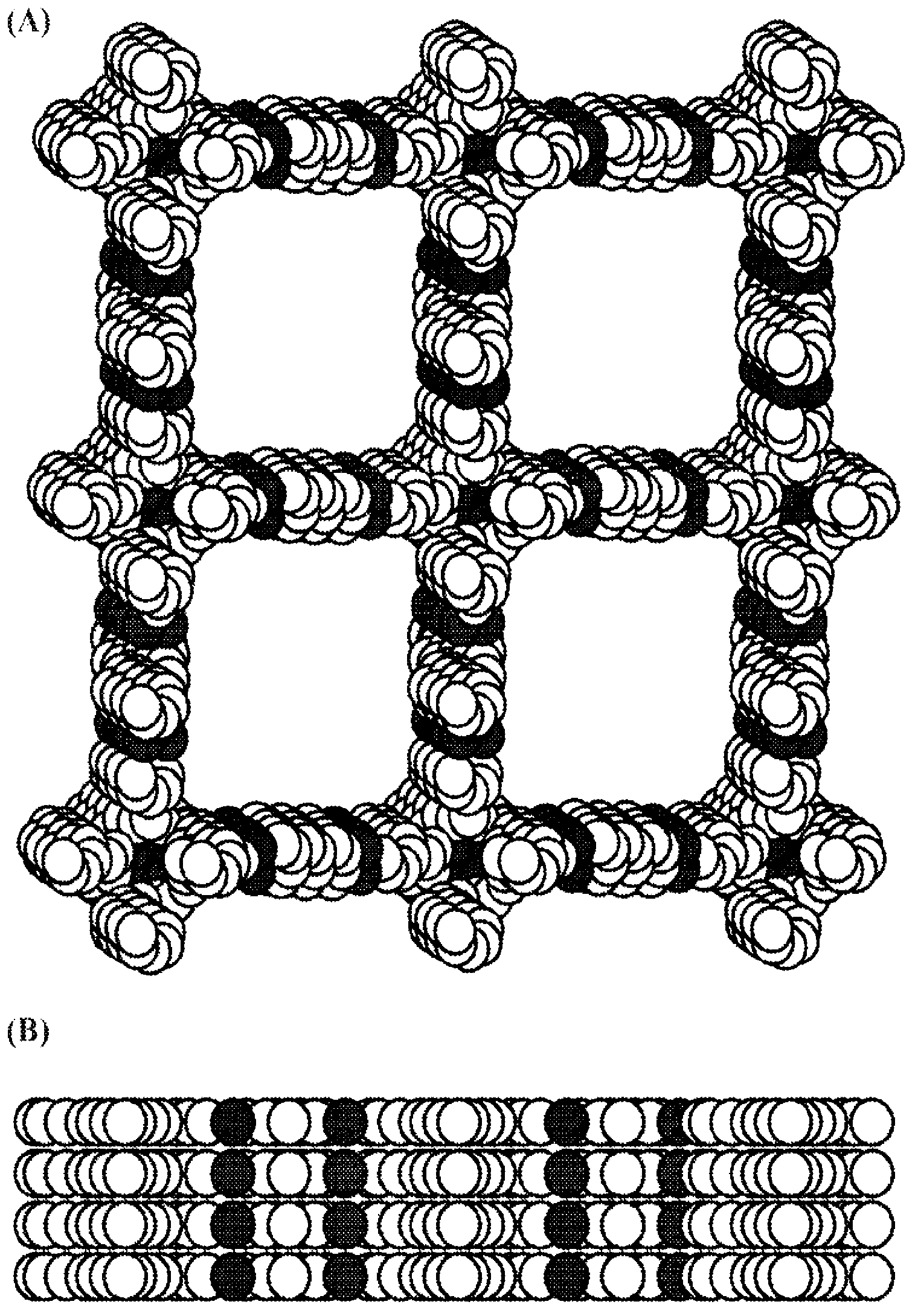
Conductive open frameworks
A conductive, covalent organic framework technology, used in non-metallic conductors, organic matter conductors, compounds containing elements of Group 3/13 of the periodic table, etc., and can solve problems such as PIM hysteresis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0305] The preparation of the disclosed frameworks can be carried out in aqueous or non-aqueous solvent systems. Solvents can be polar or non-polar or combinations thereof, as the case may be. The reaction mixture or suspension comprises a solvent system, a linker and a core. The reaction solution, mixture or suspension may also contain a catalyst. The reaction mixture can be heated at an elevated temperature or maintained at ambient temperature, depending on the reaction components.
[0306]Examples of non-aqueous solvents that can be used in the backbone-making reactions and / or as non-aqueous solvents for post-synthetic backbone reactions include, but are not limited to: n-hydrocarbon based solvents such as pentane, hexane, deca Octane, and dodecane; solvents based on branched and cyclic hydrocarbons such as cycloheptane, cyclohexane, methylcyclohexane, cyclohexene, cyclopentane; aryl and substituted aryl based solvents Solvents such as benzene, toluene, xylene, chloroben...
Embodiment
[0350] Synthetic reactions and related schemes:
[0351] All reactions were performed under argon using glove box or Schlenk line techniques. Acetone (99.8%, extra dry) was purchased from Acros Chemicals. Mesitylene (98%) was purchased from Fluka and was not dried before use. Tetrahydrofuran (HPLC grade, Aldrich) was passed through an Mbraun solvent purification system (alumina and molecular sieve columns) before use. Deuterated solvents for nuclear magnetic resonance (NMR) spectroscopic analysis (Cambridge Isotope Laboratories) were used as received. All other starting materials and solvents were obtained from Aldrich Chemical Co. and used without further purification unless otherwise stated. Analytical thin layer chromatography (TLC) on silica gel 60-F 254 (Merck5554) pre-coated glass plate carried out. Tetrakis(p-amino-phenyl)porphyrin (TAPP) and 2,3,4,5-tetrahydroxyanthracene (THAn) were synthesized using published procedures. Loaded with reagents and liquid N 2 The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com