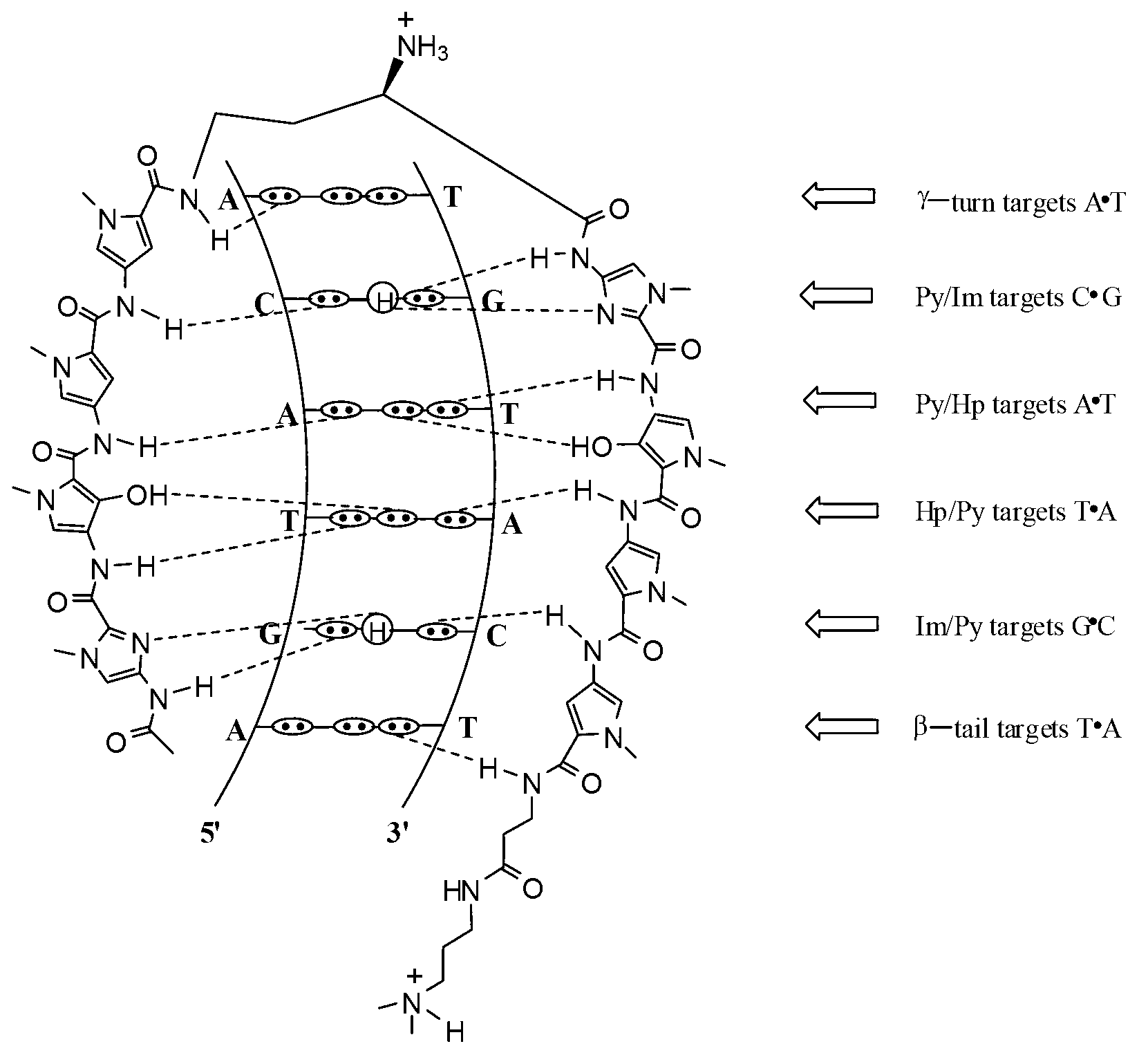
Hairpin polyamide modified by chiral (S)-beta-hydroxyl-gamma-amino acid and application for same
A hairpin polyamide and amino acid technology, which is applied to the application field of preparing antitumor drugs, can solve the problems of no polyamide regulation of c-kit gene, no specific recognition function, etc., so as to shorten synthesis time and save research and development costs. , the effect of improving the synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
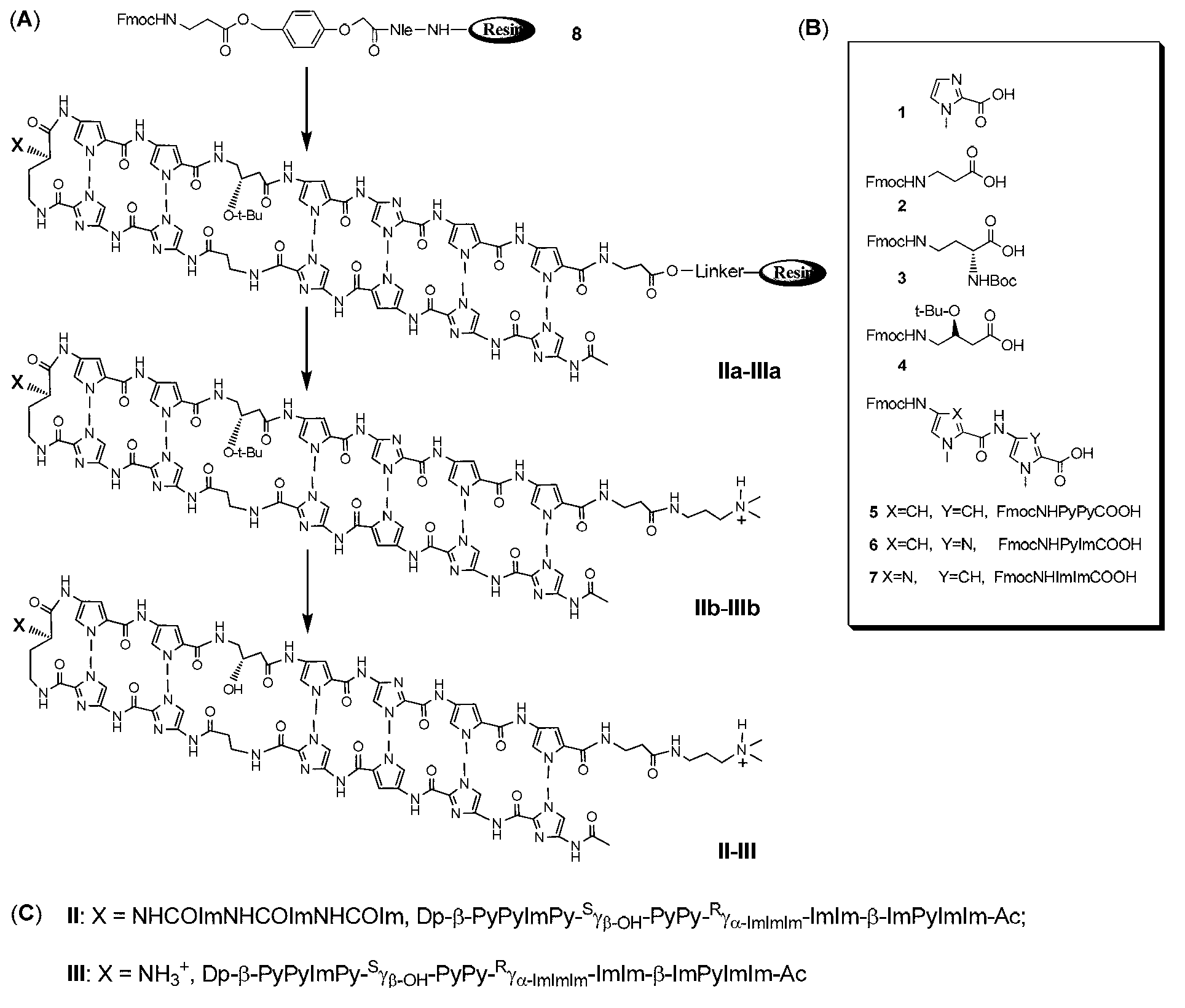
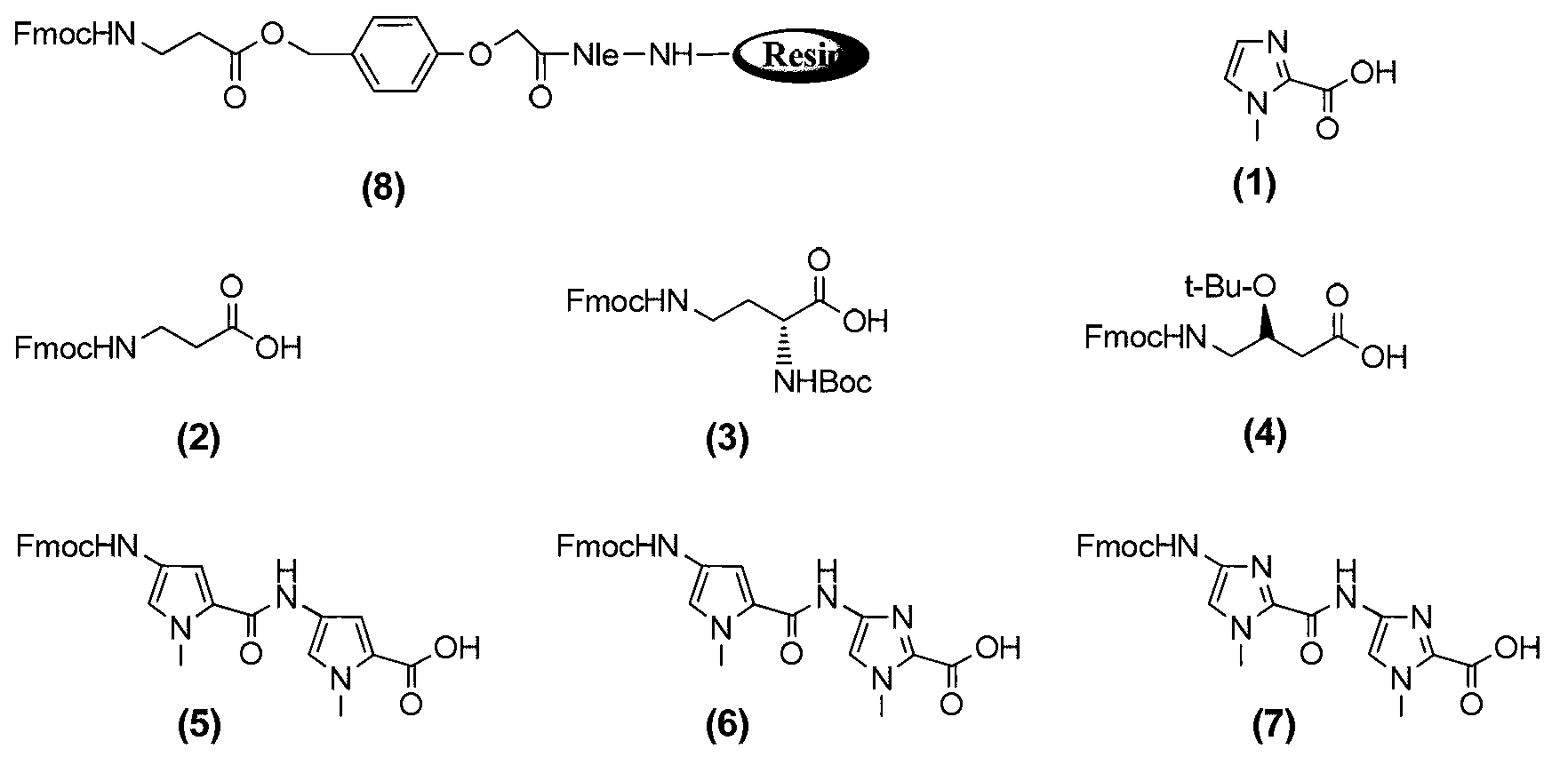
[0051] Embodiment 1: the preparation of compound II
[0052]
[0053] Table 1: Reagents and their abbreviations used in solid-phase synthesis reactions
[0054]
[0055]
[0056] Proceed as follows:
[0057] 1) Activation: Weigh the Fmoc protected β-alanine-Clear resin (SPS-1, 1.00g, 0.4mmol, Peptides International) shown in formula (8) and add it to the solid phase reaction tube of the solid phase reaction device , nitrogen protection, and then add 5mL of DMF to fully bubble nitrogen for 30min to activate the resin;
[0058] 2) Deprotection: first prepare 3 mL of 20% (v / v) piperidine / DMF (both piperidine and DMF are treated anhydrous solvents), and add it to the solid-phase reaction tube of step 1) under nitrogen protection, Fully bubbling reaction for 15 minutes, remove the amino protecting group on β-alanine, remove the solvent in the reaction tube, rinse twice with 3mL anhydrous dichloromethane, rinse once with 2mL anhydrous DMF, each time Drain the solvent af...
Embodiment 2
[0099] Embodiment 2: the preparation of compound III
[0100]
[0101] The operation steps from step 1) to step 21) are the same as in embodiment 1.
[0102] Take the intermediate (IIa=IIIa) of step 21) and the resin with the target polyamide out of the solid-phase reaction tube, put it into a reaction bottle, add 20mL of Dp, and react overnight at 55°C in an oil bath to stop the reaction. Filtrate, collect the filtrate, remove Dp under vacuum to obtain the intermediate (IIIb) of hairpin polyamide (III) protected by chiral hydroxyl and amino groups;
[0103]
[0104] Then, under the protection of nitrogen, 3 mL of deprotection reagent TFA / TMS / TIS / H with a weight ratio of 91:3:3:3 was added to the above intermediate 2 O mixed solution, react at room temperature for 1h to remove the tert-butyl group on the hydroxyl group and the tert-butoxycarbonyl group on the amino group, remove the solvent under vacuum, dissolve the product in dichloromethane, adjust to acidity with 5m...
Embodiment 3
[0105] Example 3: Native-PAGE analysis of the interaction between polyamide and c-kit gene promoter region sequence
[0106] (1) Preparation of 16% non-denaturing polyacrylamide gel (16% Native-PAGE, Table 2): used to investigate the nature of the interaction between the compound and the DNA sequence of the c-kit promoter region.
[0107] Table 2: 16% Native-PAGE recipe
[0108]
[0109]
[0110] (2) Native-PAGE electrophoresis conditions: 100V pre-electrophoresis for 30min. After adding the sample, the voltage was 150V, and the electrophoresis was stopped after 3 hours. Keep at 4°C during electrophoresis. 2 μL of SYBR Gold, stained with the staining solution in the dark for 30 minutes, and rinsed twice with deionized water. Then use the UV gel imaging system to analyze, the results are shown in Image 6 .
[0111] From the analysis of gel results: compounds II and III have obvious effects on DNA, which is concentration-dependent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com