Preparation method of carbonyl fluoride
A technology of carbonyl fluoride and sulfuryl fluoride, which is applied in chemical instruments and methods, carbon compounds, inorganic chemistry, etc., can solve the problems of high safety risk, use of large equipment, and low product purity, so as to reduce reaction heat and increase Productivity, the effect of reducing exhaust emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
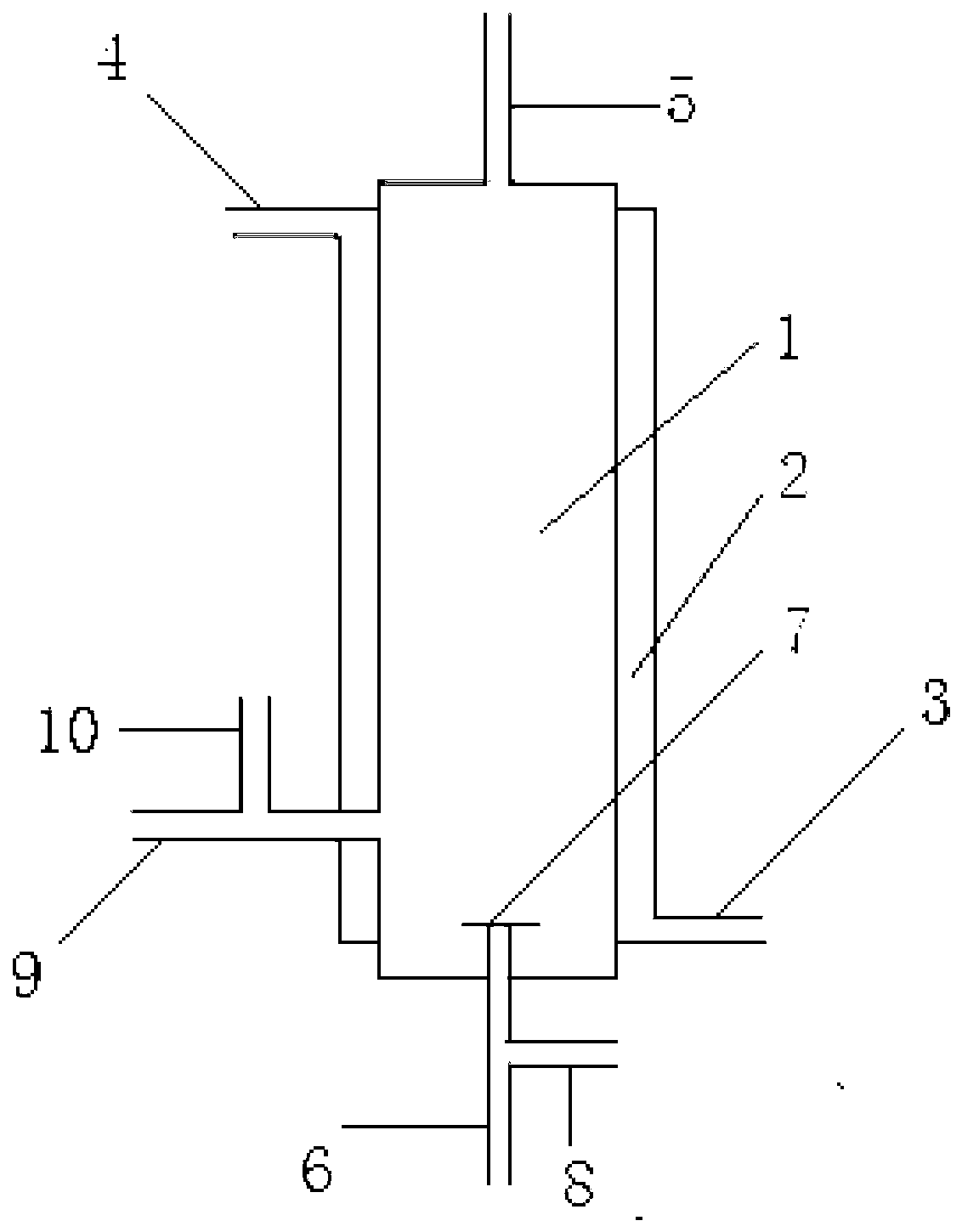
Image
Examples
Embodiment 1
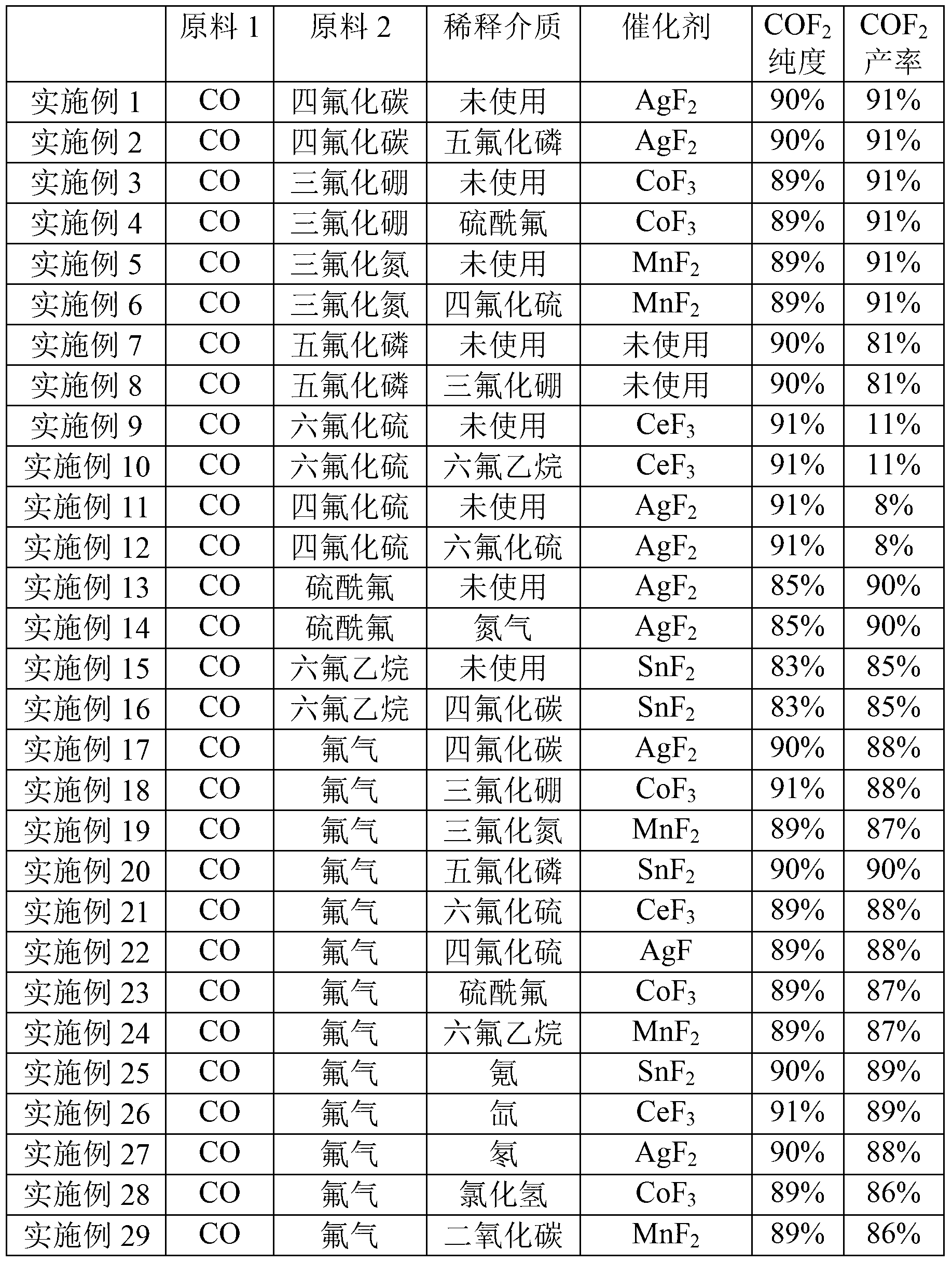
[0052] 200g of AgF 2 Put it into the reaction chamber as a catalyst, start the reaction when both the reactants and the device are in a dry state, supply carbon monoxide to the reaction chamber through the first raw material gas inlet pipe, and supply tetrafluoroethylene to the reactor through the second raw material gas inlet pipe. Carbon dioxide, calculated according to the amount converted to fluorine atoms, the gas supply flow rate under the standard state is 10m 3 / h, the ratio of fluorine atoms in carbon tetrafluoride to the amount of carbon monoxide is 20:1, at this time, the pressure of the reaction chamber is 1MPa (gauge pressure), and the reaction temperature is 500°C; The reaction product is collected by a cryogenic cold trap to obtain a collection, and the collection is vaporized to obtain a gas.
[0053] The obtained gas is tested and it can be known that COF 2 The purity is 90%, and the yield is 91%.
Embodiment 2
[0055] While reacting, phosphorus pentafluoride was supplied to the reaction chamber through the first dilution medium inlet pipe as a dilution medium, and the ratio of the amount of phosphorus pentafluoride to carbon monoxide in the dilution medium was 60:1, and the rest were the same as in Example 1.
[0056] The obtained gas is tested and it can be known that COF 2 The purity is 90%, and the yield is 91%.
Embodiment 3
[0058] 200g of CoF 3 Put it into the reaction chamber as a catalyst, start the reaction when both the reactants and the device are in a dry state, supply carbon monoxide to the reaction chamber through the first raw material gas inlet pipe, and supply trifluorine to the reactor through the second raw material gas inlet pipe. Boron, calculated according to the amount converted to fluorine atoms, the gas supply flow rate under standard conditions is 0.1m 3 / h, the ratio of fluorine atoms to carbon monoxide in boron trifluoride is 0.1:1, at this time, the pressure of the reaction chamber is 0.5MPa (gauge pressure), and the reaction temperature is 400°C; the reaction derived from the product gas outlet The product is collected by a cryogenic cold trap to obtain a collection, and the collection is vaporized to obtain a gas.
[0059] After the gas is tested, it can be known that the COF 2 The purity is 89%, and the yield is 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com