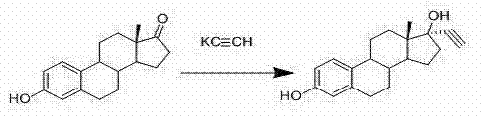
High-purity ethinyloestradiol synthesis method
A synthesis method and technology of ethinyl estradiol are applied in the field of synthesis of estrogen ethinyl estradiol, which can solve the problem that the ratio of epimers in the ethynylation product cannot be effectively controlled, and the industrial production of ethinyl estradiol cannot be satisfied, and the yield and The problem of low content, etc., to achieve the effect of easy control of generation conditions, significant comprehensive economic benefits, and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of the first step potassium alkyne:
[0036] Add 20g of potassium hydroxide powder with a water content of 13% into a three-necked reaction bottle, pass in acetylene gas to replace the air in the bottle, then seal it, then pass in normal pressure acetylene gas from the metering bottle, start stirring and heat up in a water bath, about 40°C When the inspiratory volume reaches 2.2L and the temperature reaches 75°C, the heating is stopped and cooled to room temperature under stirring to obtain the crude product of light brown powdery potassium alkyne and potassium hydroxide mixture with a potassium alkyne content of 10 %, sealed and kept for later use;
[0037] The preparation of second step ethinyl estradiol:
[0038] Add 45g of potassium alkyne powdered crude product and 45ml of THF to the three-necked reaction flask, blow out the air in the bottle with acetylene gas while stirring, keep the reaction temperature at -5~5°C, and add 5g of estrone and 0.15m...
Embodiment 2
[0040] The preparation of the first step potassium alkyne:
[0041] Add 100g of potassium hydroxide powder with a water content of 6% into a three-necked reaction bottle, pass in acetylene gas to replace the air in the bottle, then seal it, then pass in normal pressure acetylene gas from the metering bottle, start stirring and heat up in a water bath, about 40°C When the inspiratory volume reaches 12L and the temperature reaches 80°C, the heating is stopped and cooled to room temperature under stirring to obtain the crude product of light brown powdery potassium alkyne and potassium hydroxide mixture with a potassium alkyne content of 12%. , sealed and kept for later use;
[0042] The preparation of second step ethinyl estradiol:
[0043] Add 80g of potassium alkyne powdered crude product and 110ml of THF to the three-necked reaction flask, blow out the air in the bottle with acetylene gas while stirring, keep the reaction temperature at 0~10°C, add 10g of estrone and 0.15ml ...
Embodiment 3
[0045] The preparation of the first step potassium alkyne:
[0046] Put 250g of potassium hydroxide powder with a water content of 12% into a three-necked reaction bottle, pass in acetylene gas to replace the air in the bottle, then seal it, then pass in normal pressure acetylene gas from the metering bottle, start stirring and heat up in a water bath, at about 40°C Start to absorb acetylene, when the inspiratory volume reaches 24L and the temperature reaches 85°C, stop heating and cool to room temperature under stirring to obtain the crude product of light brown powdery mixture of potassium alkyne and potassium hydroxide, the content of potassium alkyne is 11%. Sealed and kept for later use;
[0047] The preparation of second step ethinyl estradiol:
[0048] Add 200g of potassium alkyne powdered crude product and 170ml of THF to the three-necked reaction flask, blow out the air in the bottle with acetylene gas while stirring, keep the reaction temperature at 0~10°C, and add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com