Method for preparing active small peptide by establishing oligopeptide concatemer yeast expression plasmid
A technology of yeast expression and active small peptides, applied in the field of bioengineering, can solve the problems of low proportion of short peptides and low yield, and achieve the effect of low development cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) In order to make the two ends of the antimicrobial peptide contain a homologous enzyme and a protein cleavage site, the enzyme cleavage sites of Xho I and Sal I were respectively introduced when designing the upstream and downstream primers of the antimicrobial peptide point. At the same time, the codon (ATG) of the cyanogen bromide (CNBr) protein cleavage site methionine (Met) was introduced at the amino terminus inside the enzyme cleavage site; the protein cleavage of hydroxylamine (NH2OH) was introduced at the carboxyl terminus. The codon AATGGA for the site (asparagine (Asn)-glycine (Gly)).
[0027] The gene coding sequence of the antimicrobial peptide is (its nucleotide sequence is shown in SEQ ID NO: 1; its amino acid sequence is shown in SEQ ID NO: 2):
[0028] aagtggaagtccttcctgaagaccttcaagtccgctgctaagactgttctgcatactgctctgaaggctatttcctcc;
[0029] Upstream primer (5'-3'):
[0030] tcgactcgagatgaagtggaagtccttcctgaagaccttcaagtccgctgctaagactgttctg;
[0031]...
Embodiment 2
[0044] (1) As in step (1) of Example 1.
[0045] (2) As in step (2) of Example 1.
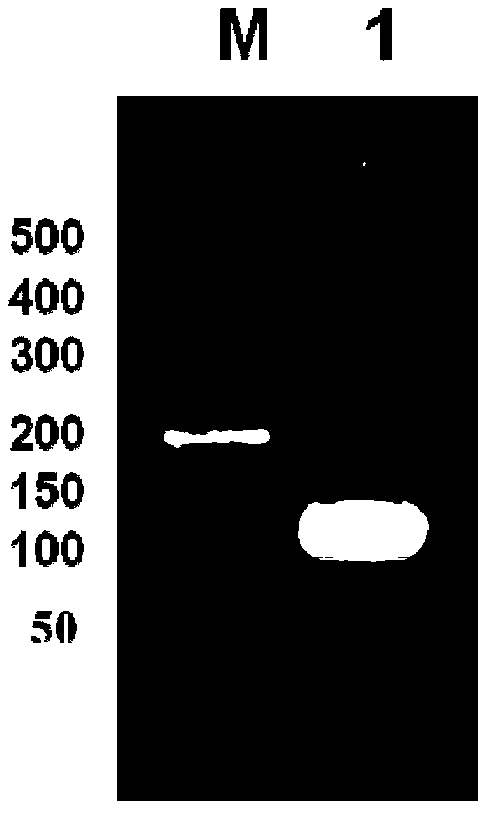
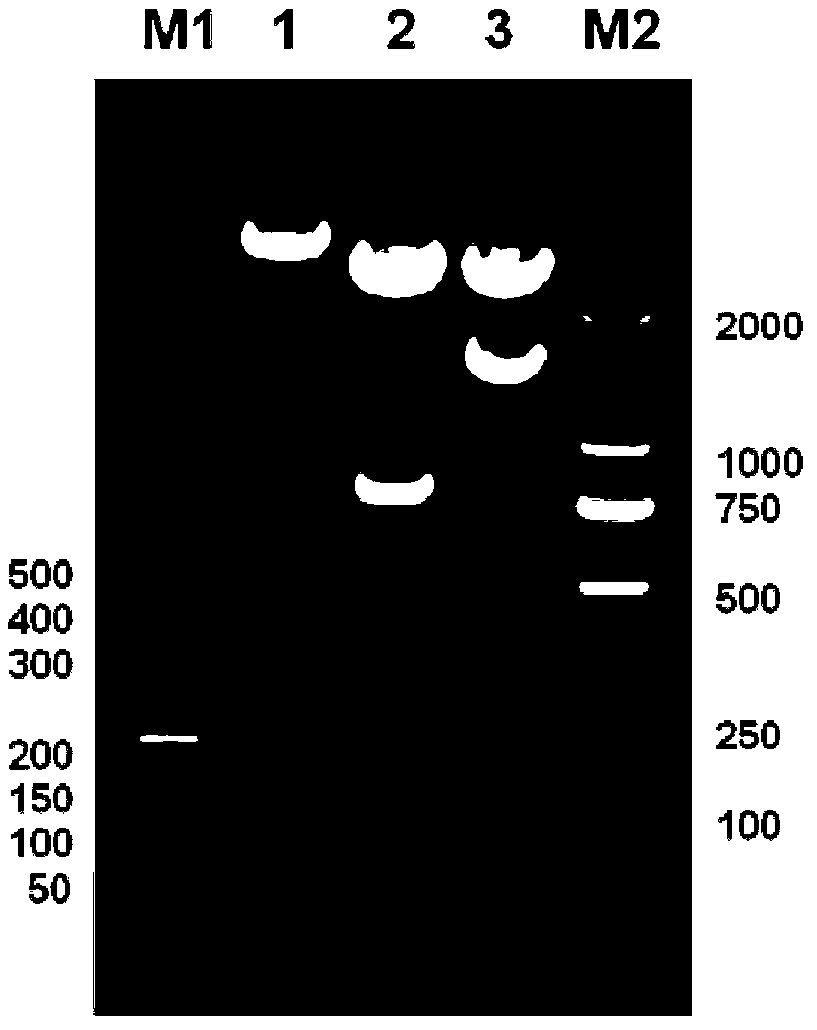
[0046] (3) The obtained pBluescriptIISK+ recombinant plasmid was transformed into Escherichia coli DH5α, a single colony was picked on a plate with an ampicillin concentration of 100 mg / L, a small amount of plasmid was extracted, double-enzyme digested and sequenced for identification. Preserve the required strains (the plasmids contain 4-fold antimicrobial peptide concatemers in correct series), and extract the plasmids containing 4-fold antimicrobial peptide concatenations from them for the construction of expression plasmids.
[0047] (4) The pBluescriptIISK+ recombinant plasmid and the empty yeast expression plasmid pPICZa were double-digested with Xho I and Xba I restriction endonucleases respectively, and agarose gel electrophoresis was performed to recover 4 times of recombinant plasmids obtained by double-digestion The antibacterial peptide concatenated fragment (the nucleotide sequenc...
Embodiment 3
[0053] (1) As in step (1) of Example 1.
[0054] (2) As in step (2) of Example 1.
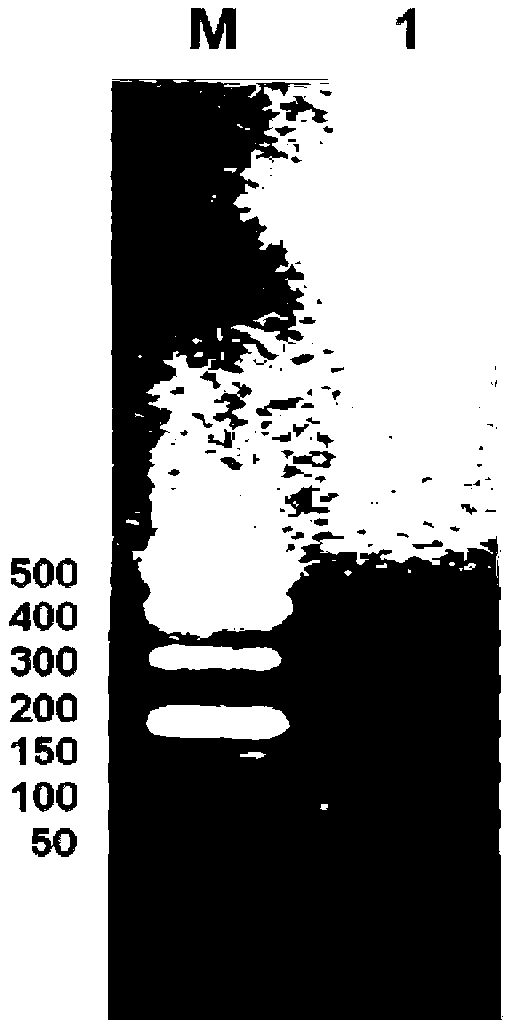
[0055] (3) The obtained pBluescriptIISK+ recombinant plasmid was transformed into Escherichia coli DH5α, a single colony was picked on a plate with an ampicillin concentration of 100 mg / L, a small amount of plasmid was extracted, double-enzyme digested and sequenced for identification. Preserve the desired strain (the plasmid contains the correct 8-fold antimicrobial peptide concatenation), and extract the plasmid containing the 8-fold antimicrobial peptide concatenation to construct an expression plasmid.
[0056] (4) The pBluescriptIISK+ recombinant plasmid and the empty yeast expression plasmid pPICZa were double-digested with Xho I and Xba I restriction endonucleases respectively, and agarose gel electrophoresis was performed to recover 8 times of recombinant plasmids obtained by double-digestion. The antimicrobial peptide concatenated fragment (its nucleotide sequence is shown in SEQ ID N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com