Silver/silver chloride electrode material, method for manufacturing same and electrode
A technology for silver chloride electrodes and electrode materials, which is applied in the direction of material analysis, analysis materials, and instruments by electromagnetic means, can solve problems such as industrial application limitations, shedding or wear, and unfavorable electrode performance, and achieve good electrochemical performance. , stable and firm adhesion, the effect of large industrialization significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Silver / silver chloride electrode paste material
[0037] Preparation of silver / silver chloride powder: Dissolve 1.2g of AgNO3 (analytically pure) in distilled water to make a silver nitrate aqueous solution with a concentration of 0.02M, and add 10g of Ag powder (average particle size 80μm) into the silver nitrate aqueous solution , stirring to disperse evenly to obtain Ag / AgNO3 dispersion system. Make 0.4g of NaCl (analytical pure) into a 0.1M aqueous solution, and add it into the Ag / AgNO3 dispersion system while stirring. After the reaction is finished, the stirring is stopped, and finally the water layer is poured off, and the reacted product is dried in an oven at a temperature of 80±5°C. In the Ag / AgCl powder prepared in this way, the mass ratio of Ag to AgCl is 1:0.1, and the converted molar ratio is 1:0.07.
[0038] Preparation of binder: Take 0.4g polyvinyl butyral, 0.2g ethyl cellulose, add 2.4g ethyl acetate to dissolve and make binder.
...
Embodiment 2
[0040] Embodiment 2: silver / silver chloride solid electrode
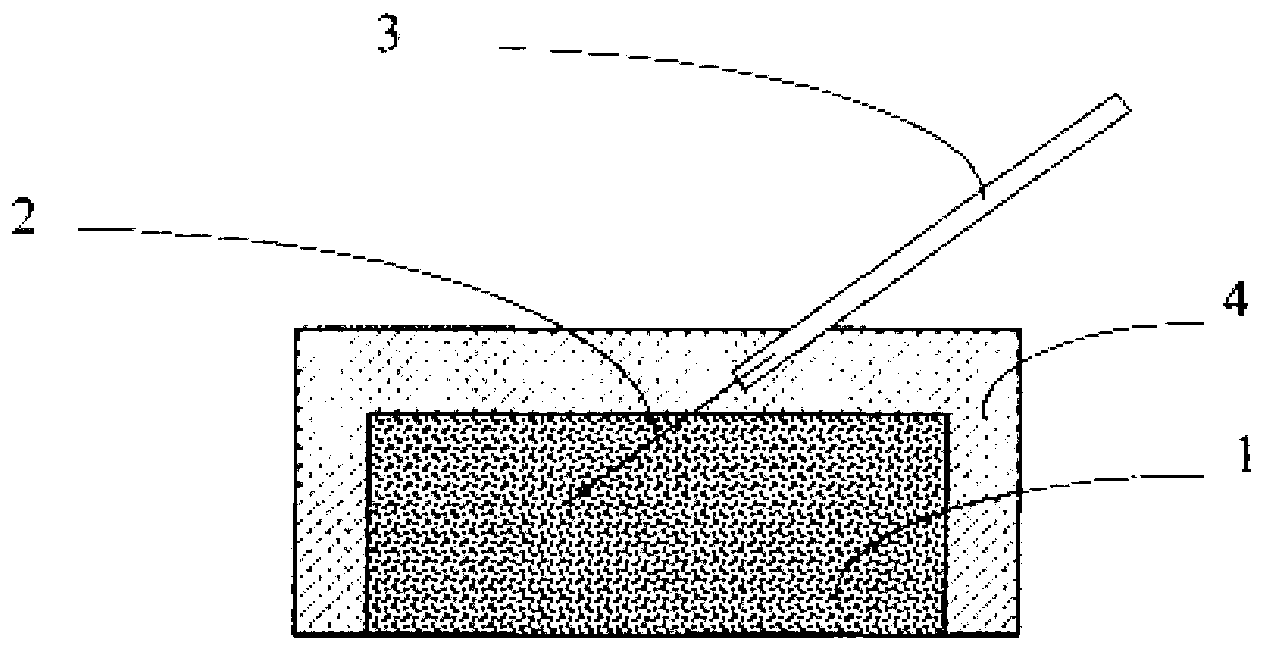
[0041] Get the silver / silver chloride slurry 1 that 0.5g embodiment 1 makes and add in the mould, combine figure 1 shown, hand-molded ( figure 1 Press into a cube) and embed silver wire 2, and then sinter at 150°C for 30 minutes to make a silver / silver chloride electrode sheet. Then the silver wire 2 is welded with the wire 3, and finally the silver / silver chloride electrode piece is encapsulated with epoxy resin 4 to obtain a finished silver / silver chloride solid electrode.
[0042]
Embodiment 3
[0043] Embodiment 3: silver / silver chloride electrode ink-like material
[0044] Preparation of silver / silver chloride powder: prepare Ag and AgCl powders below 100um respectively, weigh 3gAg and 0.3gAgCl, and convert the molar ratio of Ag to AgCl to be 1:0.07.
[0045] Preparation of binder: Take 0.4g polyvinyl butyral, 0.2g ethyl cellulose, add 2.4g ethyl acetate to dissolve and make binder.
[0046] Preparation of diluent: mix 1.3g terpineol, 1.0g ethylene glycol ethyl ether and 0.7g ethylene glycol butyl ether to prepare diluent.
[0047] Mix 3g of the above-mentioned binder, 3g of the above-mentioned diluent and 3g of the above-mentioned silver / silver chloride powder in an agate jar, and ball mill for 10 hours in a ball mill to obtain a uniformly dispersed thin silver / silver chloride ink slurry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com