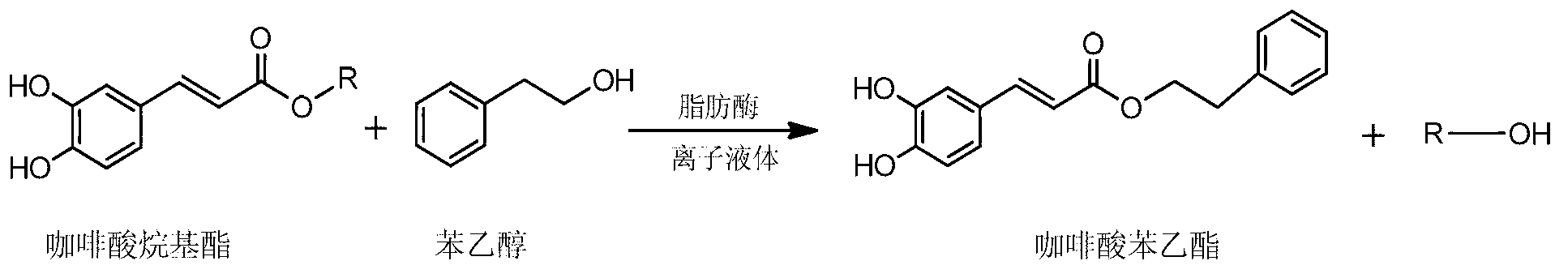
Biosynthesis method of caffeic acid phenethylester by means of transesterification
A caffeic acid phenethyl ester, biosynthesis technology, applied in the direction of fermentation, etc., to achieve the effects of less by-products, good market application prospects, and overcoming the low utilization rate of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] With caffeic acid heptyl ester as the reaction substrate, the molar ratio of caffeic acid heptyl ester: phenyl alcohol is 1:1, the mass ratio of caffeic acid heptyl ester: lipase Lipozyme TLIM is 1: 1, phenyl alcohol: ionic liquid [TOMA ][HSO 4 ] The volume ratio is 1:5 to prepare a reaction system, and react for 10 hours at 40°C and at a rotating speed of 120 rpm. After the reaction, the sample was tested by HPLC, and the yield of caffeic acid phenethyl ester was calculated to be 42.7%.
Embodiment 2
[0023] Using caffeic acid butyl ester as the reaction substrate, according to the molar ratio of caffeic acid butyl ester: phenethyl alcohol is 1:40, the mass ratio of caffeic acid butyl ester: lipase Novozym435 is 1:40, and phenyl alcohol: ionic liquid [Omim] [BF 4 ] The volume ratio is 1:50 to form a reaction system, and react for 48 hours at 90°C and 300 rpm. After the reaction, the sample was tested by HPLC, and the yield of caffeic acid phenethyl ester was calculated to be 71.7%.
Embodiment 3
[0025] Ethyl caffeate is used as the reaction substrate, and the molar ratio of ethyl caffeate: phenethyl alcohol is 1:14, the mass ratio of ethyl caffeate: industrial lipase is 1:30, and the mass ratio of phenethyl alcohol: ionic liquid [Bmim ][Tf 2 The volume ratio of N] is 1:20 to form a reaction system, and the reaction system is reacted for 40 hours at 70°C and at a rotation speed of 180 rpm. After the reaction, the sample was tested by HPLC, and the yield of caffeic acid phenethyl ester was calculated to be 63.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com