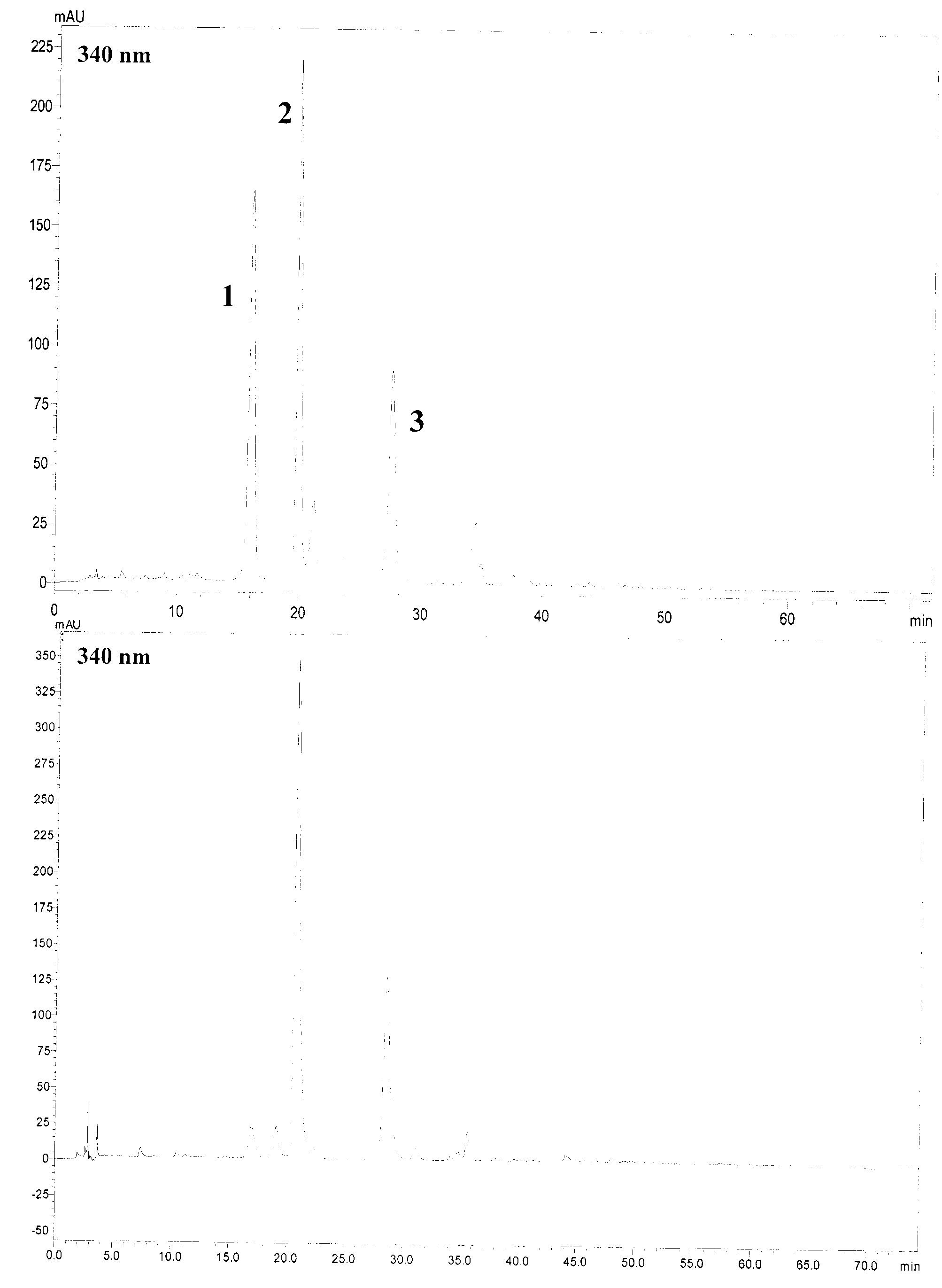Flavone C-glycoside extract and preparation method thereof
A technology of flavonoid carbon glycosides and extracts, applied in the field of medicine and health food, can solve the problems of complex composition, low product purity and total transfer rate, affecting the practical application of ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Investigation on acid hydrolysis reaction of flavonoid carbon glycoside monomer
[0018] The reference substances isoorientin and isovitexin are commercially available. Reference substance 2″-Galactosylorientin, orientin, and vitexin are self-made, and their purification process can be found in: Li Shengyin, Research on Chemical Constituents of Nasturtium, Soochow University Master’s Thesis, 2008.
[0019] Acid hydrolysis reaction: Weigh 5 mg of each reference substance, add 10 mL of 2M HCl respectively, react in water baths at two temperatures of 60°C and 80°C for 6 hours, and neutralize with 4M NaOH to pH 5-7. HPLC analysis of components such as orientin and vitexin in the hydrolyzate, chromatographic analysis conditions: ODS chromatographic column (4.6 * 250mm); Acetonitrile-0.1% acetic acid-water is mobile phase gradient elution (0-5min, 13% acetonitrile ; 5-25min, 13-15% acetonitrile; 25-30min, 15-18% acetonitrile; 30-50min, 18-28% acetonitrile; 50-6...
Embodiment 2
[0022] Example 2: Investigation on the Acid Hydrolysis Process of Trollius Component TC-A
[0023] The component TC-A of nasturtium lotus mainly contains 2″-galactosylorientin (content 30.85%), orientin (content 50.49%) and a small amount of vitexin (content 12.67%). For the purification process, please refer to: Liu Jiangyun Etc. A flavonoid carbon glycoside composition and its preparation method and application. Patent application number CN201110055655.5. The main process flow is: take 100g of dried nasturtium medicinal material, heat and reflux (1.5h) with 1000mL of water to extract twice , the extracts were combined and concentrated under reduced pressure to 1.5 g crude drug / mL, added an equal volume of 95% ethanol, stirred continuously, and filtered to obtain a filtrate; the filtrate was concentrated under reduced pressure, added water to make a sample solution of 1.0 g crude drug / mL, and AB-8 macroporous resin column, respectively eluted with 1500mL water, 2000mL30% etha...
Embodiment 3
[0032] Example 3: Investigation on the Acid Hydrolysis Process of Trollius Component TC-B
[0033] The nasturtium component TC-B mainly contains orientin esters and vitexin ester derivatives, including 2″-O-(2″′-methylbutyl) isoflavin, 2″-O- (3″′, 4″-dimethoxybenzoyl) isoflavin, 2″-O-(2″′-methylbutyl) vitexin, 2″-O-(2″′ -methylbutyl)orbitoside, 6″′-hydroxymethylglutaryl-2″-O-β-L-galactosylorbitoside, 6″′-hydroxymethylglutaryl-2″- O-β-L-galactose vitexin and so on.
[0034] Investigation of hydrolysis conditions: Weigh 1 g of TC-A sample, add 100 mL of 2.0M HCl-50% ethanol respectively, react in a water bath at 80°C for 6 hours, and neutralize to pH 5-7 with 4M NaOH. The reaction solution was further coated with AB-8 resin, and eluted with water, 30% ethanol, and 70% ethanol in sequence, and the eluted sites of 30% ethanol were collected, concentrated, and dried to obtain TC-BH product (0.56 g).
[0035] Results: Other flavone carbon glycoside derivatives in the reaction pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com