Erlotinib-hydrate crystal form I preparation method
A technology of monohydrate and erlotinib hydrochloride, which is applied in the field of preparation of erlotinib monohydrate crystal form I, can solve the problems of unsuitability for industrial scale production, cumbersome preparation methods, and easy generation of mixed crystals. Easy to recycle and reuse, strong operability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of erlotinib monohydrate crystal form I
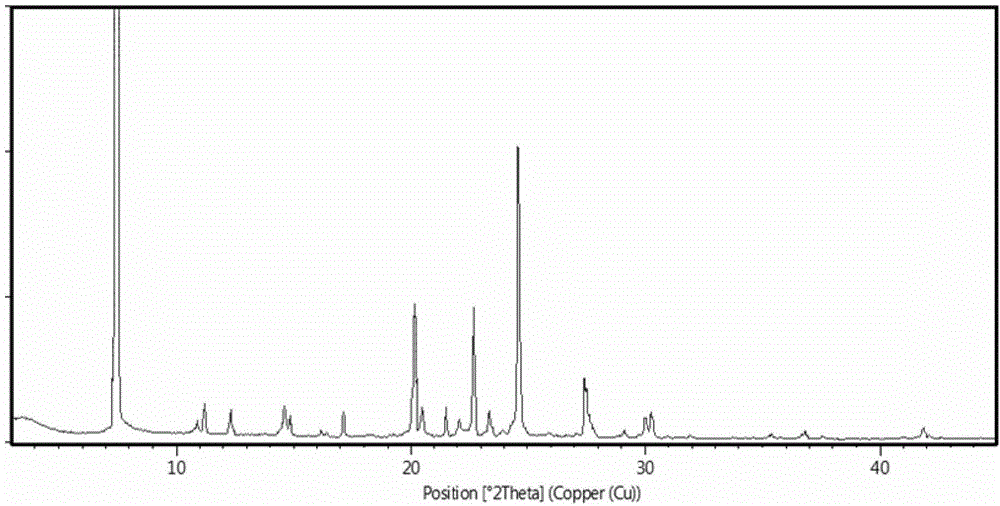
[0030] Erlotinib hydrochloride 6.0g (14mmol), add 30ml of n-butanol, add 1.0mol / L potassium hydroxide aqueous solution 15ml, heat to 80 ℃ under stirring, the solid dissolves completely, let stand and separate, discard the water phase, Separate the organic phase while it is hot, and naturally cool the organic phase to room temperature while stirring, continue to stir for 1 hour, filter with suction, and dry the filter cake at 50-60°C to obtain the white crystal of erlotinib monohydrate crystal form I Sexual solid 5.21g, yield 86.8%, HPLC purity is 99.7%, measure the X-PRD that obtains and figure 1 Basically the same.
Embodiment 2
[0031] Embodiment 2: Preparation of erlotinib monohydrate crystal form I
[0032] Add 6.0g of erlotinib hydrochloride, add 36ml of n-butanol, add 14ml of 2.0mol / L sodium hydroxide aqueous solution, heat to 85°C under stirring, until the solid is completely dissolved, let stand to separate layers, discard the water phase, and Separate the organic phase, and naturally cool the organic phase to room temperature while stirring, continue to stir for 1 hour, filter with suction, and dry the filter cake at 40-50°C to obtain erlotinib monohydrate crystal form I as a white crystalline solid 5.14g, yield 85.7%, HPLC purity 99.8%.
Embodiment 3
[0033] Embodiment 3: Preparation of erlotinib monohydrate crystal form I
[0034] Add 6.0g of erlotinib hydrochloride, add 48ml of n-butanol, add 1.0mol / L potassium carbonate aqueous solution 15ml, heat under stirring until the temperature reaches 70°C, the solid is completely dissolved, let stand to separate layers, discard the water phase, and The organic phase is separated by heat, and the organic phase is naturally cooled to room temperature under stirring. After continuing to stir for 2 hours, it is filtered with suction, and the filter cake is dried at 50-60°C to obtain the white crystalline form of erlotinib monohydrate The solid was 5.03g, the yield was 83.8%, and the HPLC purity was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com