Secondary lithium-air battery cathode catalyst
A cathode catalyst, air battery technology, applied in battery electrodes, fuel cell type half cells and secondary battery type half cells, circuits, etc., can solve the problem that transition metal oxides have poor conductivity, are easily terminated, cannot be Take advantage of electrocatalytic performance and other issues to achieve the effects of excellent catalytic efficiency, reduced production costs, and long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
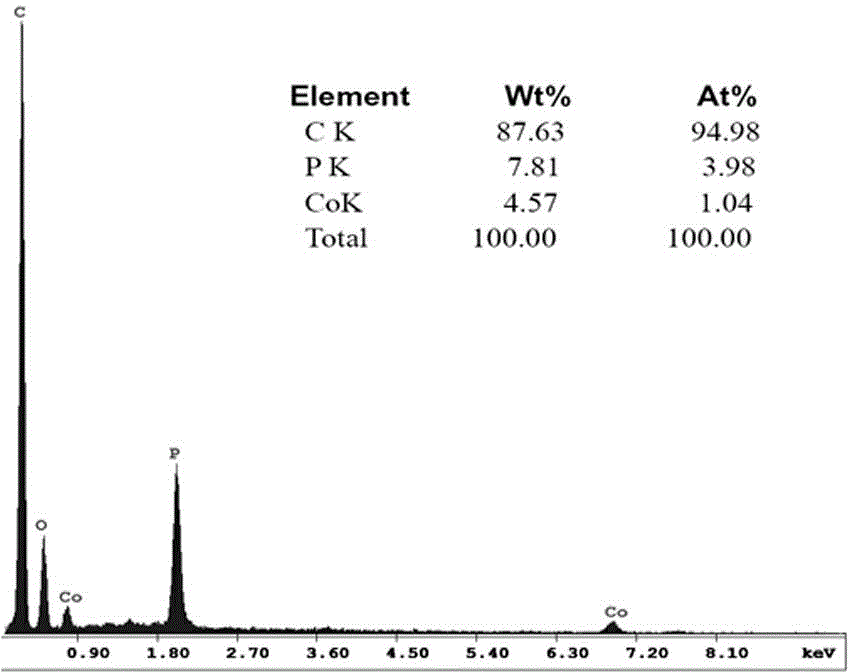
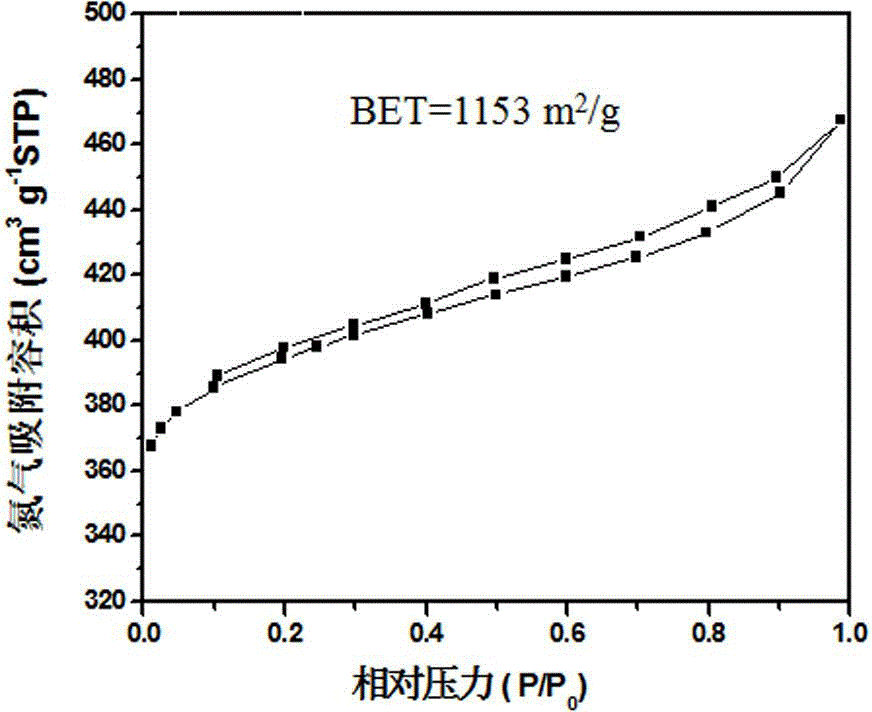
[0034] Example 1: Preparation of P and Co co-doped porous carbon
[0035] Weigh resorcinol and formaldehyde, mix, the molar ratio of resorcinol and formaldehyde is 1: 2, and add 50ml deionized water, vigorously stir to form a homogeneous solution, then add Co(NO 3 ) 2 ·6H 2 O, make the molar ratio of resorcinol and Co 1:20, and stir to dissolve; then add ammonia water dropwise to make the solution form a sol.
[0036] The above sol was dried in a vacuum oven at 85° C. for 7 days to form a gel; the gel was heat-treated in a tube furnace at 1000° C. for 2 h under an Ar atmosphere to obtain Co-doped porous carbon. Phosphoric acid was added to the obtained Co-doped carbon, so that the mass ratio of carbon to phosphoric acid was 1:10, and impregnated at 80 °C for 2 h, then the phosphoric acid and carbon were separated, and the obtained carbon material was heated in a tube furnace under an Ar atmosphere. Heating at 800°C for 1 h, then cooling to room temperature naturally, P and ...
Embodiment 2
[0042] Example 2: Preparation of P and Fe co-doped porous carbon
[0043] Put 1M sucrose solution into an autoclave with a volume of 100ml and seal it with a filling degree of 70 v%, heat the autoclave in an oven at 220°C for 12 hours to obtain a carbon intermediate; weigh the carbon intermediate and add 0.1M Ferric chloride solution, so that the molar ratio of Fe and carbon intermediates is 0.01:1, soak for 3 hours, then dry at 100°C; then add phosphoric acid, make the mass ratio of carbon and phosphoric acid 1:16, soak for 3 hours at 80°C , and then the phosphoric acid was separated from the carbon, and the resulting carbon material was heated in a tube furnace at 900 °C for 2 h in an Ar atmosphere to obtain P and Fe co-doped porous carbon. The molar ratio of carbon is 3:97, and the molar ratio of P and Fe is 2:1.
[0044] A button battery was prepared and tested. First, it was discharged to 2.0V at 30mA / g, and then charged to 4.2V. The released capacity was calculated base...
Embodiment 3
[0045] Example 3: Preparation of P, Fe and Co co-doped porous carbon
[0046] Weigh resorcinol and formaldehyde and mix, so that the molar ratio of resorcinol and formaldehyde is 1:2, and add 100 ml deionized water, stir vigorously to form a homogeneous solution, then add Co(NO 3 ) 2 ·6H 2 O and ferric chloride, make the molar ratio of Fe and Co 1:1~3, preferably 1:3, make the molar ratio of resorcinol and Co 1:15, stir and dissolve; then add ammonia water drop by drop , so that the solution forms a sol.
[0047] The above sol was dried in a vacuum oven at 85°C for 10 days to form a gel, which was heat-treated in a tube furnace at 1000°C for 3 h under an Ar atmosphere to obtain Co and Fe co-doped porous carbon. Phosphoric acid was added to the obtained Co and Fe co-doped carbon, so that the mass ratio of carbon to phosphoric acid was 1:12, and immersed at 80 ° C for 3 h, then the phosphoric acid and carbon were separated, and the obtained carbon material was heated in a tub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com