Kit for detecting genotype of human chromosome 21 STR (short tandem repeat)
A technology for detecting reagents and chromosomes, applied in the field of molecular biology, can solve the problems that QF-PCR technology has not yet been applied, and achieve high diagnostic efficiency, high detection sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Application of the kit for detecting the STR genotype of human chromosome 21 in the detection of the number of chromosome 21
[0034] (1) Kit composition (100 people) (Table 3).
[0035]
[0036] (2) Main instruments and equipment
[0037] PCR amplification instrument, ABI310 genetic analyzer, high-speed centrifuge, biological safety cabinet or clean bench, micro pipette, ultraviolet spectrophotometer, constant temperature water bath, refrigerator, etc.
[0038] (3) DNA extraction
[0039] The sample is 0.2ml of fresh EDTA anticoagulated whole blood or 10ml of amniotic fluid, DNA is extracted by Chelex-100 method (Chelex reagent is Bia-rad product), the purity and concentration of DNA are detected by UV spectrophotometer, and the extracted sample DNA is added to pure water Dilute to a concentration of about 0.1ng / μL-0.5 ng / μL.
[0040] (4) PCR reaction system (25 μL) (Table 4).
[0041]
[0042] (5) PCR cycle parameters: 95°C for 2 minutes - (9...
Embodiment 2
[0049] Example 2: Rapid prenatal diagnosis of trisomy 21 with this kit
[0050] 558 remaining amniotic fluid samples (amniotic fluid volume 0.5-1ml) analyzed by conventional karyotype were used in the kit of the present invention for blind detection and analysis according to Example 1. A total of 5 cases of trisomy 21 (all complete types, no chimeras and translocations) were detected in 558 amniotic fluid samples by this kit, and the test results can be obtained within 48 hours for each sample.
[0051] Compared with the results of chromosome karyotype diagnosis, all trisomy 21 was detected by D21 trisomy analysis fluorescent detection kit, without missing detection and amplification failure, with 100% diagnostic sensitivity and 100% diagnostic accuracy. From the perspective of the detection time of a single sample, the time-consuming use of the D21 trisomy analysis fluorescent detection kit is only 1 / 10 of the traditional karyotype analysis method. Due to the high-throughp...
Embodiment 3
[0053] Example 3: Determining the source of extra chromosomes in trisomy 21 embryos
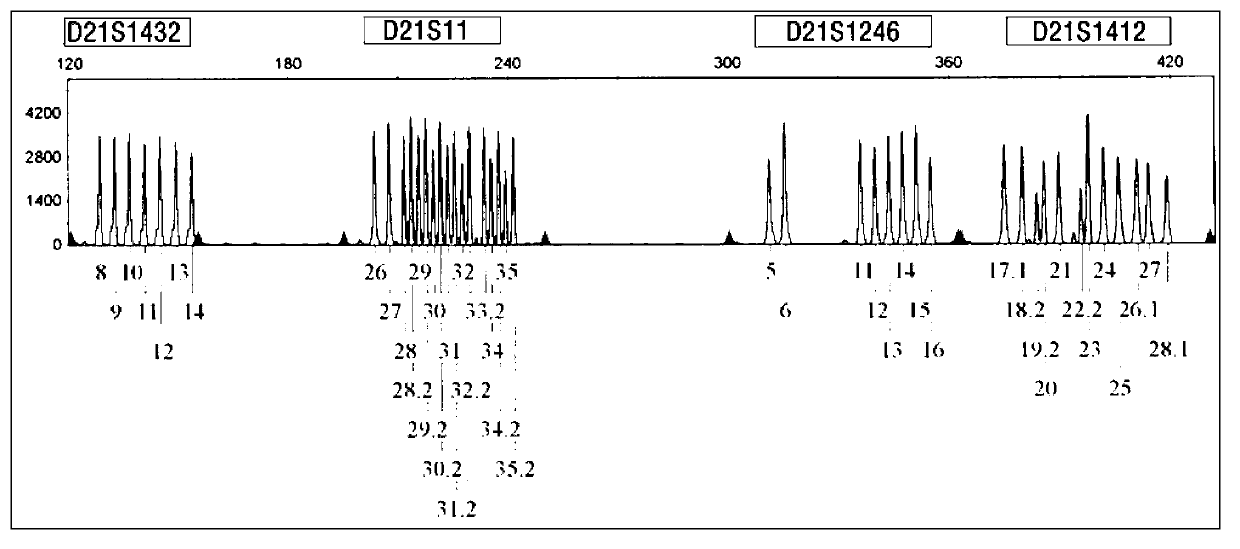
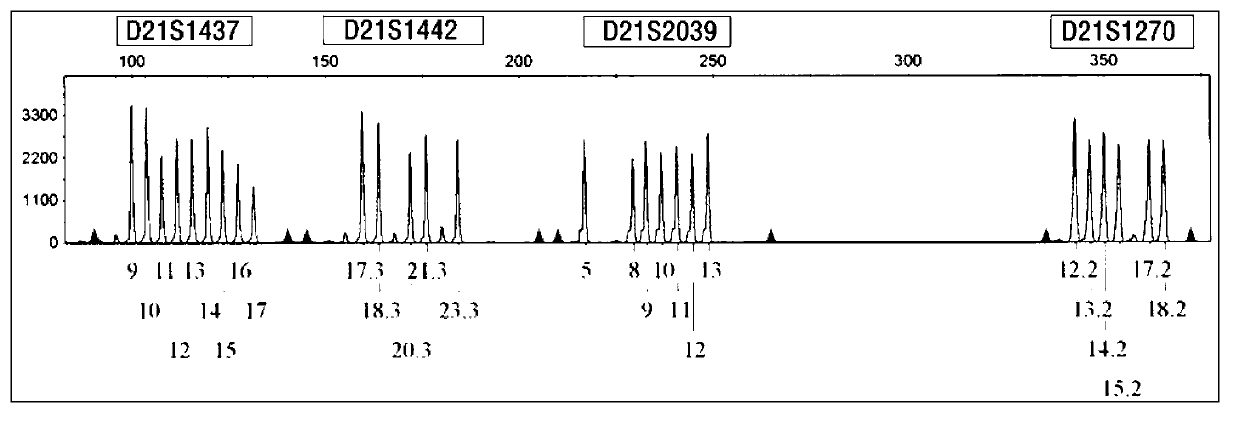
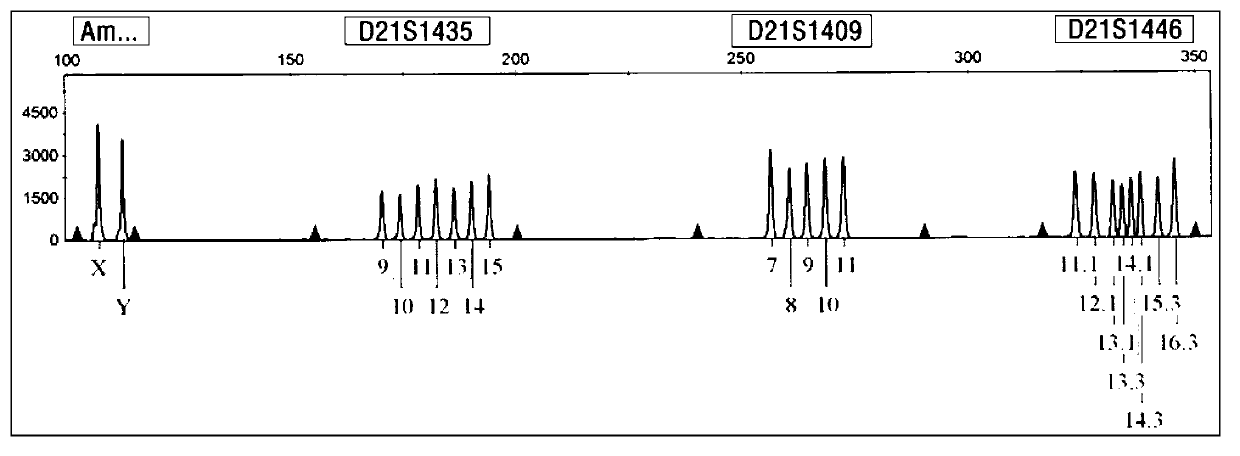
[0054] Collect 6 aborted villi tissues (about 2 g each) known to be trisomy 21 karyotype (already done karyotype analysis), and extract peripheral blood samples from the father and mother of the aborted embryo (2ml EDTA per case) Anticoagulant), use the kit of the present invention to carry out blind detection and analysis according to the implementation case 1. The results show that this kit can clearly determine the relative source of redundant chromosomes through detection, which is helpful for guiding the next pregnancy. attached Figure 5-a~5-c It is the analysis of the source of redundant chromosomes in trisomy 21 aborted embryos. It is the genotype detection of one family at the D21S11 and D21S1246 loci. Since the alleles of the embryos come from the parents, the genotype comparison shows that the embryo samples are at D21S11 , The redundant allele on the D21S1412 locus comes f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com