Synthesis method of metoprolol succinate
A technology for the synthesis of metoprolol succinate, which is applied in the field of medicinal chemistry, can solve the problems of low yield of synthetic routes, environmental pollution, etc., and achieve the effects of simple operation, environmental friendliness and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
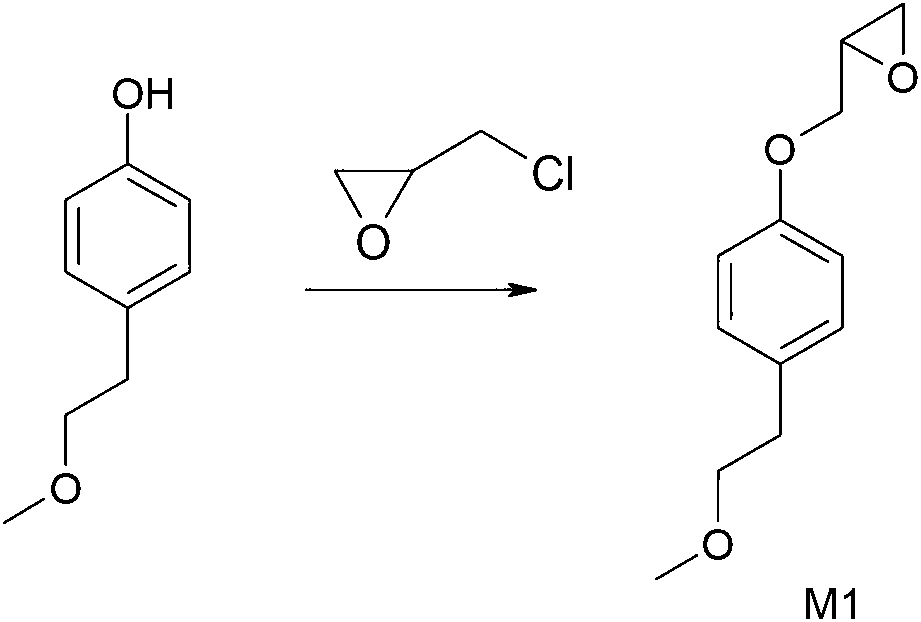
[0052] A. Preparation of 3-[4-(2-methoxyethyl)phenoxy]-1,2-propylene oxide (compound M1)
preparation example A-1
[0054] Put 40L of purified water and 2.31Kg of sodium hydroxide into a 100L glass reactor, and stir until the solids are fully dissolved. Weigh 8Kg of 4-(2-methoxyethyl)phenol, add it into a glass reactor, and stir for 30 minutes. Measure 4.86Kg of epichlorohydrin, add it into a glass reactor, heat and stir for 10 hours, and control the internal temperature at 30°C. After the reaction was completed, the heating was stopped, and 16 L of ethyl acetate was added to the glass reactor for extraction. The organic phase was dried over anhydrous magnesium sulfate. Filter and distill the filtrate under reduced pressure with a rotary evaporator. 10.27Kg of 3-[4-(2-methoxyethyl)phenoxy]-1,2-propylene oxide (M1) was obtained with a yield of 93.8%.
preparation example A-2
[0056] Put 40L of purified water and 2.31Kg of sodium hydroxide into a 100L glass reactor, and stir until the solids are fully dissolved. Weigh 8Kg of 4-(2-methoxyethyl)phenol, add it into a glass reactor, and stir for 30 minutes. Measure 5.35Kg of epichlorohydrin, put it into a glass reactor, heat and stir for 6 hours, and control the internal temperature at 40°C. After the reaction was completed, the heating was stopped, and 16 L of ethyl acetate was added to the glass reactor for extraction. The organic phase was dried over anhydrous magnesium sulfate. Filter and distill the filtrate under reduced pressure with a rotary evaporator. 10.42Kg of 3-[4-(2-methoxyethyl)phenoxy]-1,2-propylene oxide (M1) was obtained with a yield of 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com