Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
A simultaneous detection and respiratory technology, applied in microorganism-based methods, biochemical equipment and methods, and microbial determination/inspection, etc. Sensitivity and specificity, avoiding the effects of low specificity and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
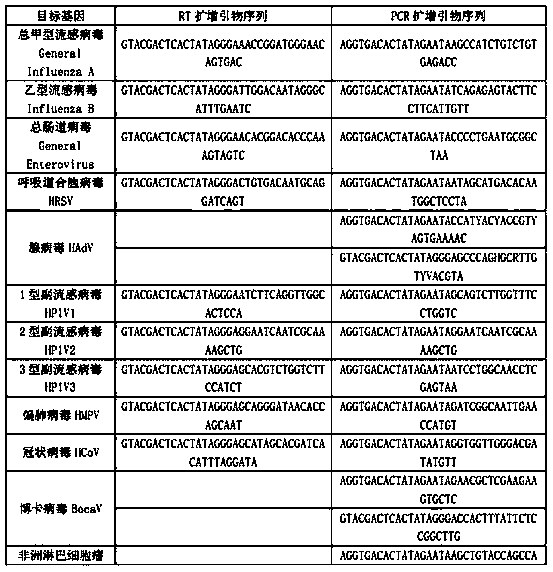
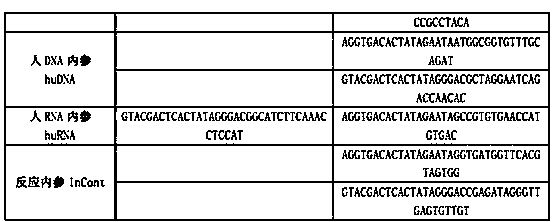
[0036] The present invention is a kit for simultaneous detection of twenty-two kinds of respiratory pathogens, including DEPC water, 5×RT buffer, reverse transcription primer (RT primer mix), RT enzyme (full name reverse transcriptase), X solution, 10 ×PCR buffer, PCR primers, 25mM magnesium chloride solution, DNA polymerase, positive control substance (cloned from each target pathogen, including the target fragment plasmid), 5×RT buffer, 10×PCR buffer, 25mM magnesium chloride solution, DNA polymerase was ordered from sigma company. The reverse transcription primers include the RT amplification primers of nine kinds of respiratory pathogens in the following table 1 and the RT amplification primers of the internal reference of human RNA, and the above PCR primers include the forward and reverse PCR amplification primers of the remaining 13 respiratory pathogens in the following table 1 , the forward and reverse PCR amplification primers of the internal reference of human DNA, t...
specific Embodiment 2
[0046] The invention is a detection method for synchronously detecting 22 kinds of respiratory pathogens, the detected respiratory pathogens include total influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus, type 1 parainfluenza virus, type 2 parainfluenza virus Viruses, parainfluenza type 3, metapneumovirus, coronavirus, bocavirus, African lymphoma virus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, group A streptococcus, haemophilus influenzae Bacillus, Streptococcus pneumoniae, Legionella, Mycobacterium tuberculosis, Mycoplasma pneumoniae, Chlamydia pneumoniae, etc. (see Table 1), collect patient samples (nasopharyngeal swab, sputum, etc.), extract nucleic acid, and use patient nucleic acid as a template for reverse transcription React with PCR, and finally separate the sample by capillary electrophoresis, the specific steps are as follows:
[0047] 1. Production of a kit for synchronously detecting 22 kinds of respiratory ...
specific Embodiment 3
[0076] Detection Kit Sensitivity and Specificity Analysis
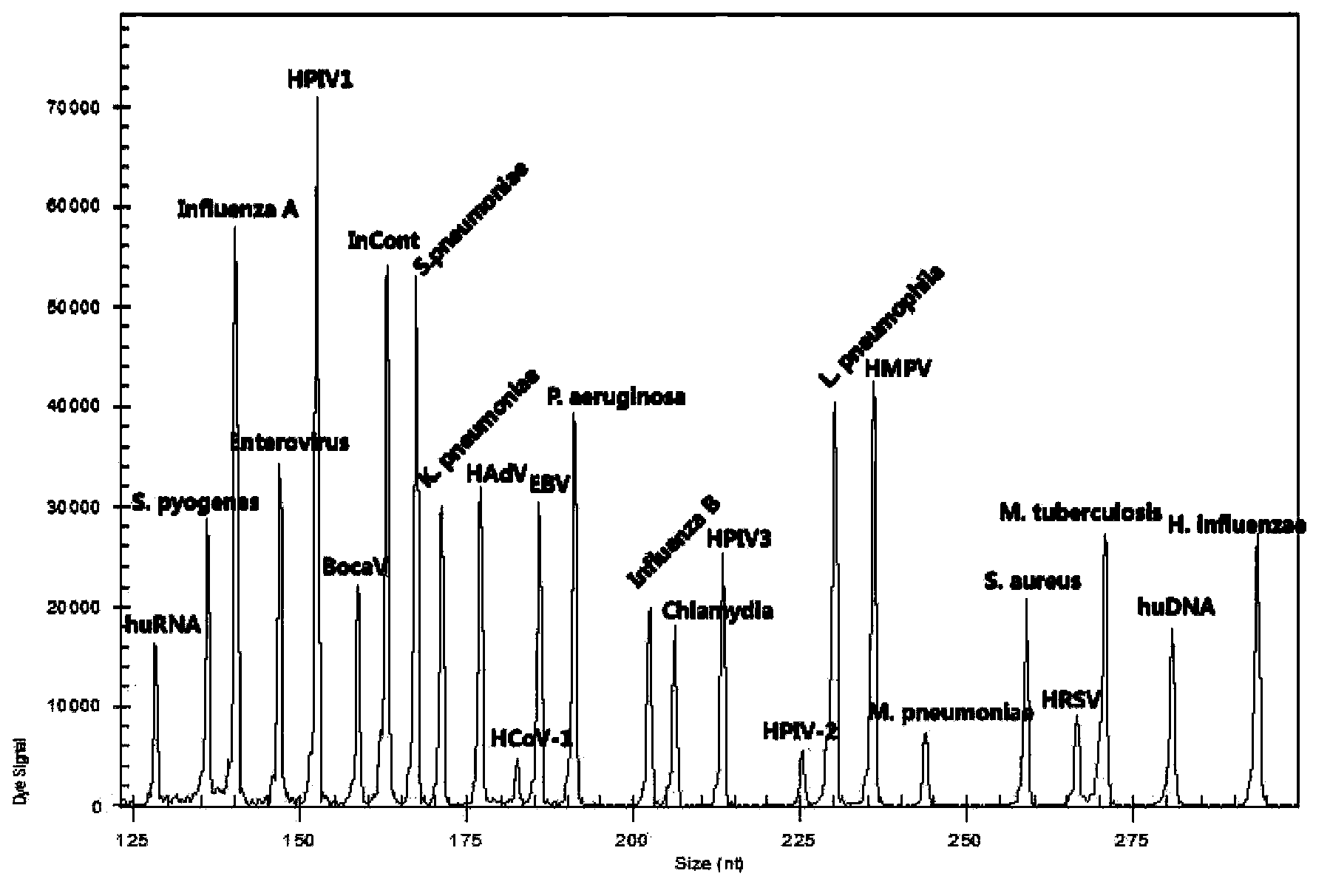
[0077]Sensitivity analysis: After diluting the positive control according to a certain copy number ratio, it is detected by PCR amplification and capillary electrophoresis until no signal is detected. The copy number is the lowest detection line, which is the sensitivity of the kit. The highest sensitivity can detect 40 copies.
[0078] Specificity analysis: Single-plex PCR amplification is detected as a single peak of the target fragment size by capillary electrophoresis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com