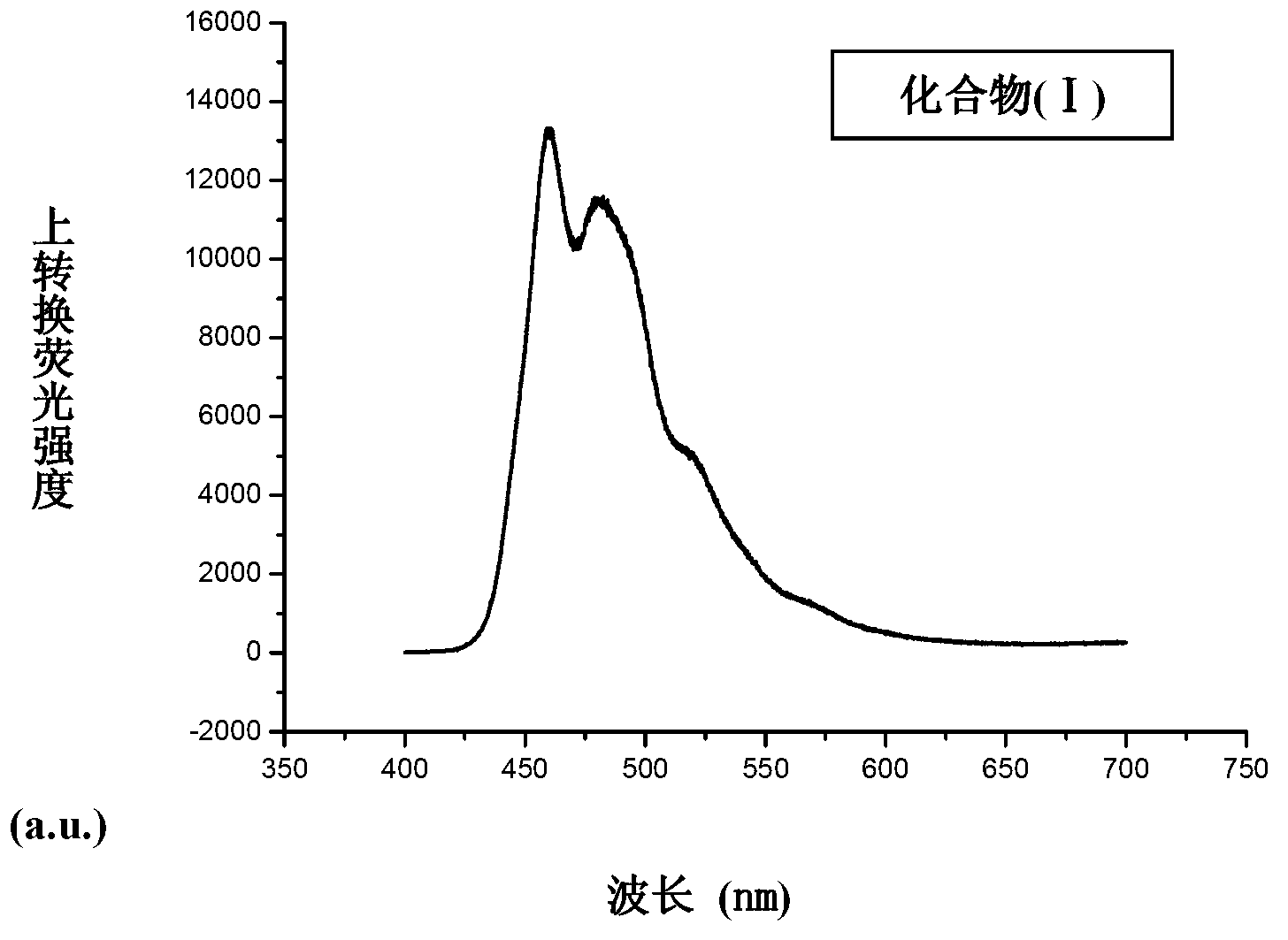
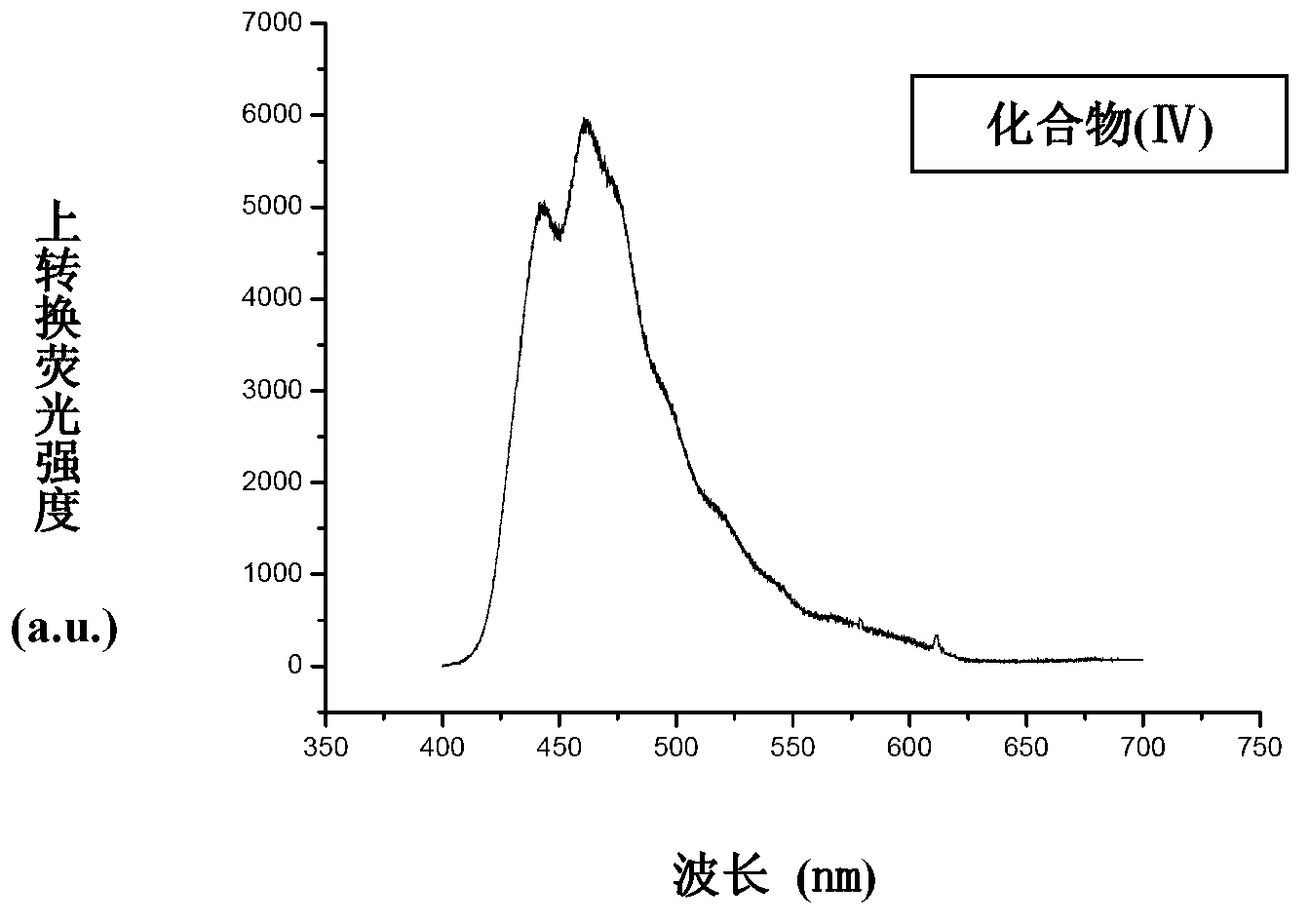
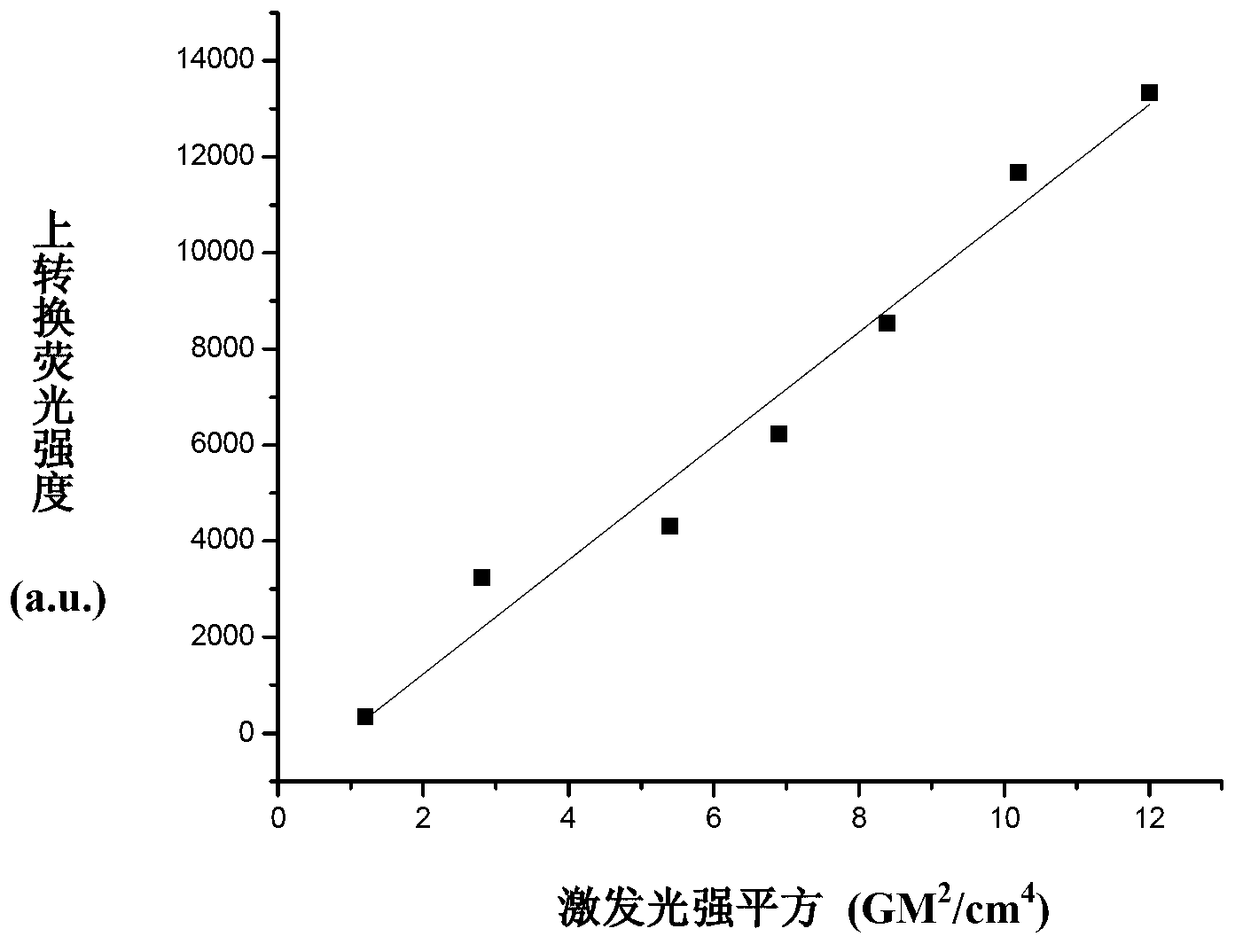
Application of phenylene bis-vinylidene bis-benzimidazole compound
A technology of phenylenedivinylene and bisbenzimidazole is applied in the field of phenylenedivinylidene bisbenzimidazole compounds, which can solve problems such as large differences, reduce energy difference, and enhance electron cloud delocalization. degree, the effect of increasing the transition moment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Compound (Ⅲ)
[0021] Heat 8.28g (60mmol) of p-xylylene dimethyl ether, 8.10g (270mmol) of paraformaldehyde and 150mL of acetic acid to 60°C, and then add 40mL of 40% mass percent hydrobromic acid solution. React at 60°C for 4 hours, cool, filter the precipitated solid, dissolve it with dichloromethane, wash it with saturated sodium bicarbonate solution several times, and distill off the solvent to obtain 18.75 g of white 1,4-bis(bromomethyl)- 2,5-Dimethoxybenzene. 1 H NMR (500MHz, CDCl 3 ) δ (ppm): 6.89 (s, 2H), 4.55 (s, 4H), 3.89 (s, 6H).
[0022] 12.62g (90mmol) of hexamethylenetetramine and 9.72g (30mmol) of 1,4-bis(bromomethyl)-2,5-dimethoxybenzene were dissolved in 200mL of chloroform, heated to reflux for 4h, and the solvent was evaporated 200mL of 50% acetic acid solution was added to the obtained solid, heated to reflux for 5h, and then a small amount of concentrated hydrochloric acid was added dropwise. After cooling, the mixture was extracted wi...
Embodiment 2
[0023] Example 2 Compound (I)
[0024] Dissolve 7.92 g (0.06 mol) of 2-methylbenzimidazole (Ⅱ) and 10 g of potassium hydroxide in 50 mL of N,N-dimethylformamide, add dropwise 3.88 g (0.02 mol) of compound (Ⅲ) in 30 mL solution in N,N-dimethylformamide. After dropping, heated to reflux for 12 h, cooled to room temperature, a large amount of solid precipitated out, filtered with suction, dried, and separated by silica gel column chromatography to obtain 6.08 g of yellow compound (I). 1 H NMR (500MHz, DMSO-d 6 ) δ (ppm): 13.23(s, 2H), 7.97(d, 2H), 7.57~7.59(m, 4H), 7.44(s, 2H), 7.41(d, 2H), 7.22~7.25(m, 4H ), 3.99(s, 6H). ESI-MS m / z: 423.2 ([M+H] + ). Elem. Anal. Calcd for C 26 h 22 N 4 o 2 : C 73.92, H 5.25, N 13.26; Found: C 74.13, H 5.31, N 13.47.
Embodiment 3
[0025] Example 3 Compound (I)
[0026] Dissolve 6.60 g (0.05 mol) of 2-methylbenzimidazole (Ⅱ) and 8 g of sodium ethoxide in 60 mL of methanol, and add dropwise a solution of 3.88 g (0.02 mol) of compound (Ⅲ) in 60 mL of methanol. After dropping, heated to reflux for 20 h, cooled to room temperature, a large amount of solid precipitated out, filtered with suction, dried, and separated by silica gel column chromatography to obtain 5.32 g of yellow compound (I).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com