Electrochemical method for simultaneous determination of tetrachlorocatechol and tetrachlorohydroquinone based on graphene/chitosan-modified electrode
A technology of tetrachlorocatechol and modified electrodes, which is applied in the field of electrochemical analysis to achieve the effects of good selectivity, good stability and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of electrochemical method for simultaneously measuring tetrachlorocatechol and tetrachlorohydroquinone based on graphene / chitosan modified electrode comprises the following steps:
[0034] 1. Preparation of graphene-chitosan suspension
[0035] a1: Measure 2.50 mL of 0.50 mg / mL graphene oxide dispersion, add 2.50 mL of twice distilled water, 20.00 μL of ammonia water, and 3.00 μL of hydrazine hydrate in sequence, vibrate vigorously, then place in a water bath at 98 °C, keep stirring for 80 min That is, the graphene suspension is obtained.
[0036] a2: Measure 1.00 mL of the above graphene suspension, add 30.00 μL of 3% chitosan, and sonicate for 30 min to obtain a stable graphene-chitosan suspension, which is sealed and stored for later use.
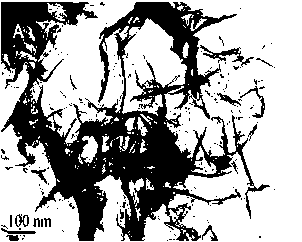
[0037] Select a small amount of prepared graphene-chitosan suspension to carry out the transmission electron microscope experiment, the gained transmission electron microscope picture is as follows figure 1 shown. From ...
Embodiment 2
[0051] Embodiment 2: the selection of buffer solution kind
[0052] According to the electrochemical determination method in the embodiment 1, three kinds in sodium acetate-acetic acid, B-R, sodium dihydrogen phosphate-citric acid (pH is 4.5) when comparing tetrachlorocatechol and tetrachlorohydroquinone coexist Electrochemical response in the buffer solution, it is found that the peak potential difference between tetrachlorocatechol and tetrachlorohydroquinone is not large in three buffer solutions, but the peak shape is the best in sodium acetate-acetic acid buffer solution, Therefore, it is recommended to choose sodium acetate-acetic acid (ie ABS) as the test base solution.
[0053]
Embodiment 3
[0054] Embodiment 3: the optimization of graphene-chitosan modifier dosage
[0055] According to the method of Example 1, graphene / chitosan modified electrodes with different graphene-chitosan modifier dosages (2-8 μL) were prepared, and the cycle voltage of tetrachlorocatechol and tetrachlorohydroquinone were studied respectively. The relationship between the oxidation peak current and the amount of graphene-chitosan modifier, when the amount of modifier is less than 5.00 μL, the peak current increases with the increase of the amount, and after more than 5.00 μL, the peak current gradually decreases, which may be Because the dosage increased continuously at the beginning, the enrichment efficiency increased, resulting in an increase in the peak current, and then with the increase in the amount of modification, the thickness of the modified film on the surface of the glassy carbon electrode continued to increase, thereby affecting the conductivity of the electrode. Therefore, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com