Escherichia coli and method for efficiently secreting and expressing human epidermal growth factor by using same
A technology for Escherichia coli and epidermal growth factor, which is applied in the field of Escherichia coli and its high-efficiency secretion and expression of human epidermal growth factor, can solve the problem of low expression level, achieve high expression level, and avoid bacterial physiology Effect of state change and plasmid loss, reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
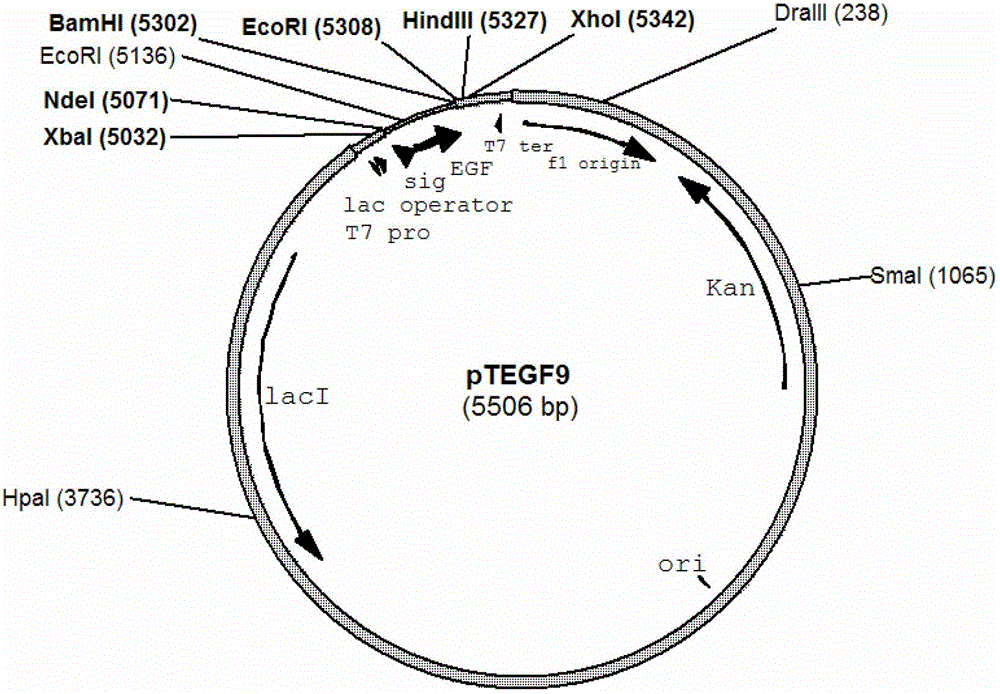
[0047] Example 1 Construction and identification of expression plasmid pTEGF9
[0048] For routine molecular experiment operation methods, refer to "Molecular Cloning Experiment Guide" (Science Press, third edition). With EGF-1 (5'-AAA CATATG AAACAAAGCACTATTGCACTG-3', the underlined part is the restriction site introduced) and EGF-2 (5'-AAA GGATCC TTATTAACGCAGTTCCCACC-3') was used as primer, and plasmid pE-5 was used as template to amplify the hEGF gene fragment with phosphatase signal peptide. The amplified gene fragment was digested with BamHI and NdeI. NdeI double-digested plasmid pET30a was connected and transformed into E. coli In TOP10 competent cells, spread on LB plates containing 30 μg / ml kanamycin, culture overnight, pick colonies and use EGF-2 and EGF-3 (5'-TACCATCGACACCACCCACGC-3') as primers for colonization PCR, verified by agarose gel electrophoresis, verified that the correct plasmid was through DNA sequencing, and the correct plasmid was named pTEGF9 (...
Embodiment 2
[0049] Example 2 Construction of expression strains, screening of high expression strains and detection of plasmid stability
[0050] 2.1 Construction of expression strains and screening of high expression strains
[0051] The plasmid pTEGF9 was treated with CaCl 2 Transform method into E. coli BL21(DE3) competent cells were spread on LB plates containing 30 μg / ml kanamycin, cultured overnight at 37°C, and 100 positive clones were picked and inoculated into 10 mL liquid containing 30 μg / ml kanamycin In LB medium, 37°C, 220 rpm, culture for 36 hours, take 0.5 mL of the culture solution, centrifuge to get the supernatant, put it into a vacuum rotary evaporator for concentration, and concentrate to 50 μl. Use the Tricine-SDS-PAGE method for electrophoresis (this gel has three layers, from top to bottom are 5% stacking gel, 10% interlayer gel and 15% separating gel). After electrophoresis, use high-sensitivity Coomassie brilliant blue Staining method Stain and decolorize, sc...
Embodiment 3
[0056] Example 3 Shake flask culture of Escherichia coli (Escherichia coli) BTE-9 (ie E. coli BL21(DE3) / pTEGF9) expressing rhEGF
[0057] Shake flask culture:
[0058] The medium formula and culture method were optimized by single factor analysis and uniform experimental design. Culture in a 250 mL shake flask containing 50 mL of medium, and the ingredients in each 50 mL of medium are:
[0059] Polypeptone 0.5 g Yeast extract 0.4 g
[0060] Glucose 0.4 g Potassium dihydrogen phosphate 0.04 g
[0061] Dipotassium hydrogen phosphate 0.1 g Ammonium sulfate 0.1 g
[0062] Anhydrous magnesium sulfate 0.025 g Glycine 0.05 g
[0063] Saturated phenol red 100 μl Kanamycin 30 μg / mL
[0064] 1% (v / v) of the frozen strains were inoculated in the shake flask culture medium, the culture temperature was 37°C, the rotation speed was 220 rpm, and the culture was continued for 35 hours, during which the pH was adjusted to 7.0.
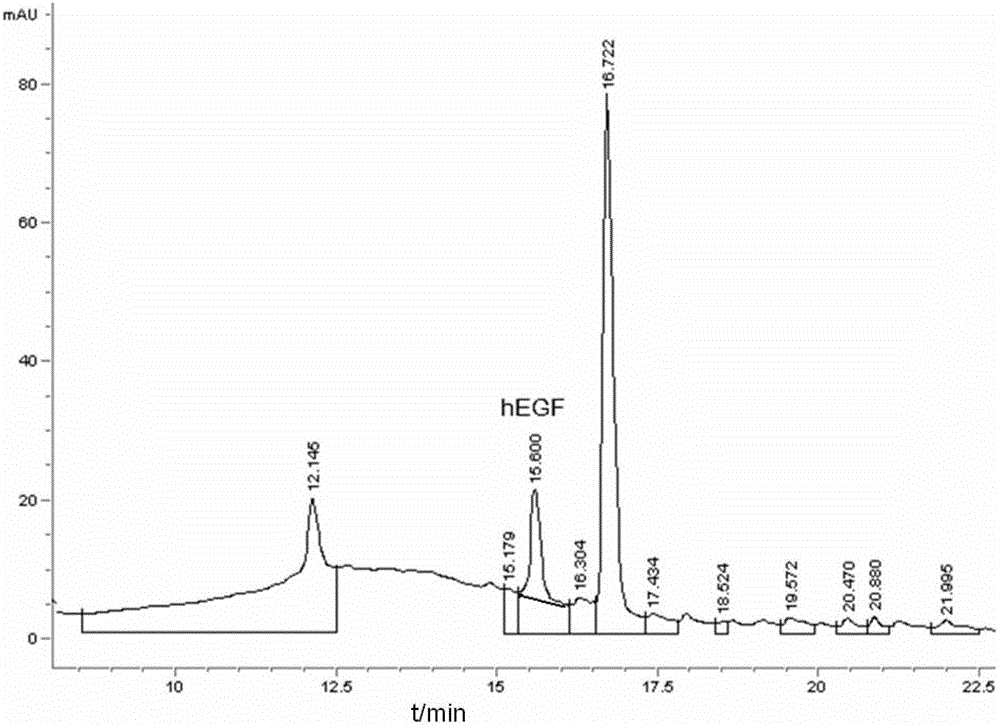
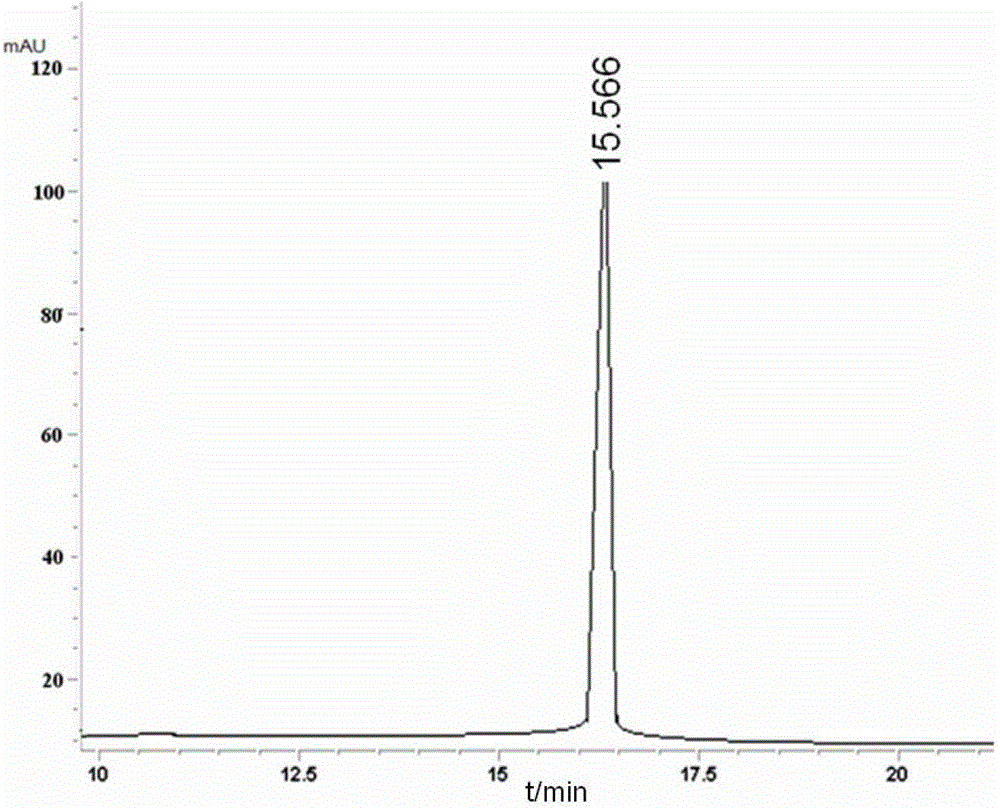
[0065] During the culture process, samples were taken e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com