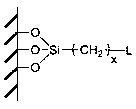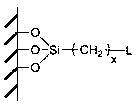Novel class of calixarene crown ether bond-type silicon-based adsorption materials and preparation method thereof
An adsorption material, hydrocarbon crown ether technology, applied in the direction of selective adsorption, chemical instruments and methods, other chemical processes, etc., can solve the problems of reducing the stability and reuse efficiency of adsorption materials, loss and decomposition, weak binding force, etc., to achieve excellent Thermal and chemical stability, avoidance of bleed, improved force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of Si-3-Calixcrown
[0047] Weigh 3.5 g of diamino-substituted bis(2-propoxy)calix[4]arene-crown-6 and dissolve it in 100 mL of tetrahydrofuran (THF), add 8.7 g of 3-chloropropyl-containing silicon-based precursor , and added 14.3 g K 2 CO 3 and 6.1 g KI. Keep stirring and under nitrogen atmosphere, reflux at 85°C for 12 h. The solid particles were separated by filtration, and washed repeatedly with deionized water and ethanol to remove salt ions. Recycling of unreacted bonded diaminobis(2-propoxy)calix[4]arene-crown-6 monomer. The obtained adsorption material particles are dried under vacuum condition, and the solvent is removed to obtain the final product.
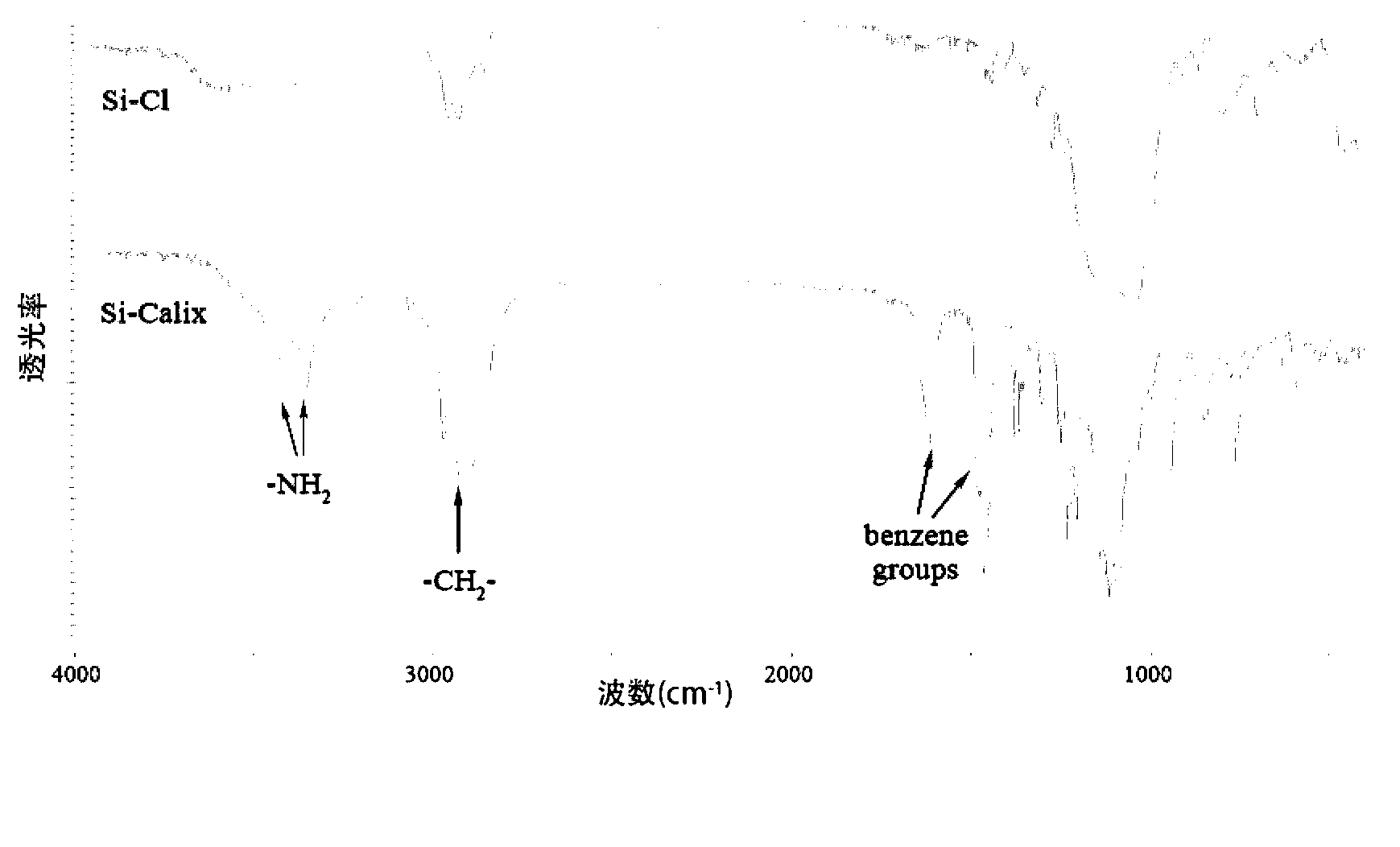
[0048] The infrared spectrum of the prepared calixarene crown ether bonded silicon-based material is shown in figure 1 shown. By comparison, it can be clearly seen that in the spectrum of the bonding material, the wave numbers are 3426, 3349 cm -1 There are obvious amine-based a...
Embodiment 2
[0049] Embodiment 2: the synthesis of Si-5-Calixcrown
[0050] Dissolve 5.7 g of diaminobis(2-propoxy)calix[4]arene-crown-6 in 80 mL of N,N-dimethylformamide (DMF), add 12.5 g of 5-bromopentyl Silicone precursors. The catalysts are NaOH and NaI, and the addition amounts are NaOH8.5 g and NaI6.4 g, respectively. Under the protection of nitrogen gas, the reaction was stirred and refluxed at 100°C for 10 h. After the reaction, the resin particles were separated by filtration. Unreacted bonded diaminobis(2-propoxy)calix[4]arene-crown- enters the filtrate, which can be recycled. The solid resin particles are repeatedly washed with deionized water and ethanol to completely elute the corresponding salt ions and entrained crown ether monomers, and then vacuum-dried to remove the solvent to obtain the final product.
Embodiment 3
[0051] Embodiment 3: the synthesis of Si-7-Calixcrown
[0052] Weigh 9.6 g of diaminobis(2-propoxy)calix[4]arene-crown-6 and dissolve it in 150 mL of N,N-dimethylacetamide, add 20.3 g of silicon containing 7-iodoheptyl base precursor, and added 4.8 g NaH. Keep stirring and under nitrogen atmosphere, react at 75°C for 8 h. The resin particles were separated by filtration, and washed repeatedly with deionized water, ethanol, and hot acetone to remove salt ions. Recycling of unreacted bonded diaminobis(2-propoxy)calix[4]arene-crown-6 monomer. The obtained solid particles are dried under vacuum conditions, and the solvent is removed to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com