Enzyme-linked immunoassay kit and method for nitrofural metabolite detection
A technology of nitrofurazone and metabolites, which can be used in the field of enzyme-linked immunoassay detection and can solve problems such as carcinogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of antigens, antibodies and enzyme markers
[0042] 1. Synthesis of Furacilin Metabolite Hapten
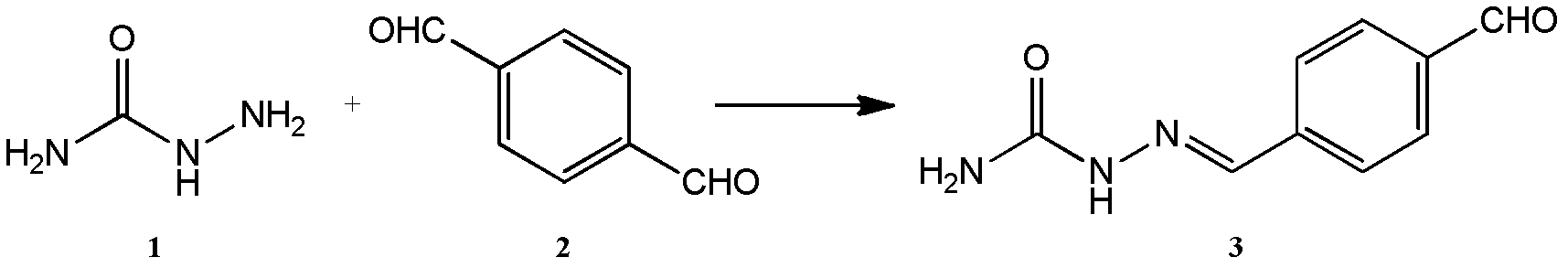
[0043] The mixture of 0.75g nitrofurazone metabolite (SEM) and 20ml DMF was slowly added dropwise at room temperature to 50-100ml DMF solution of 2.68-5.36g terephthalaldehyde, and reacted at room temperature to 60°C for 2-4 hours after the addition was completed. The solvent was removed and purified by column chromatography to obtain a pale yellow SEM derivative.
[0044] 2. Synthesis of Immunogen
[0045] (1) Take 10 mg of nitrofurazone metabolite hapten and dissolve it in 1 ml of DMF to obtain solution 1.
[0046] (2) Dissolve 40 mg of BSA in 6 ml of water to obtain solution 2.
[0047] (3) Add solution 1 dropwise to solution 2 to obtain solution 3, and react at room temperature for 24 hours.
[0048] (4) Take NaBH 4 14mg was dissolved in 0.2ml of 0.1M NaOH and added to solution 3, and reacted at 4°C for 2h.
[0049] (5) Dialyze with 0.01mol / l...
Embodiment 2
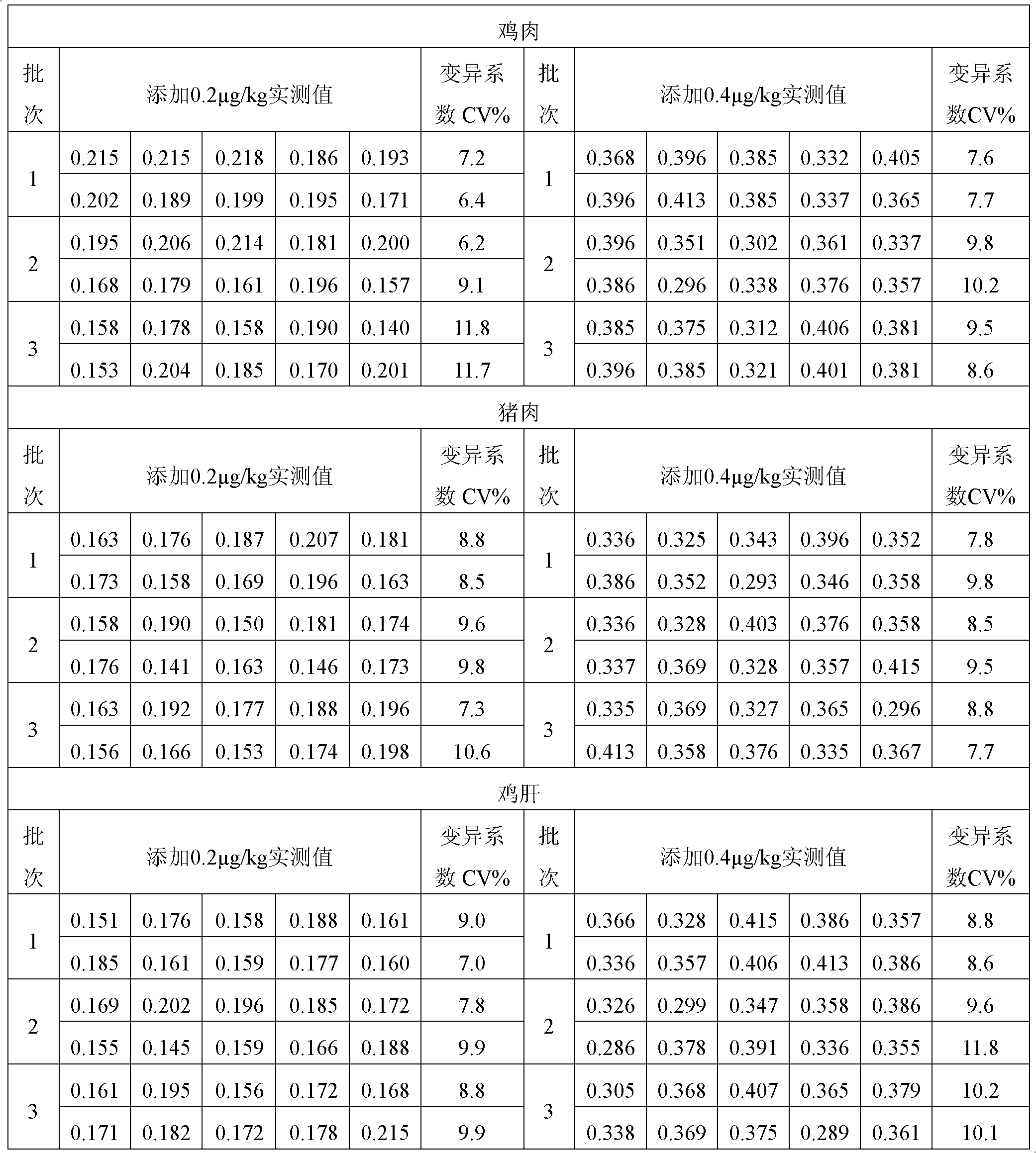
[0070] Example 2: The composition of each component of the nitrofurazone metabolite ELISA kit
[0071] Set up the nitrofurazone metabolite ELISA kit, including the following components:
[0072] (1) A microtiter plate coated with a coating source.
[0073] (2) Enzyme-labeled anti-antibody: horseradish peroxidase-goat anti-mouse anti-antibody.
[0074] (3) Working solution of nitrofurazone metabolite monoclonal antibody.
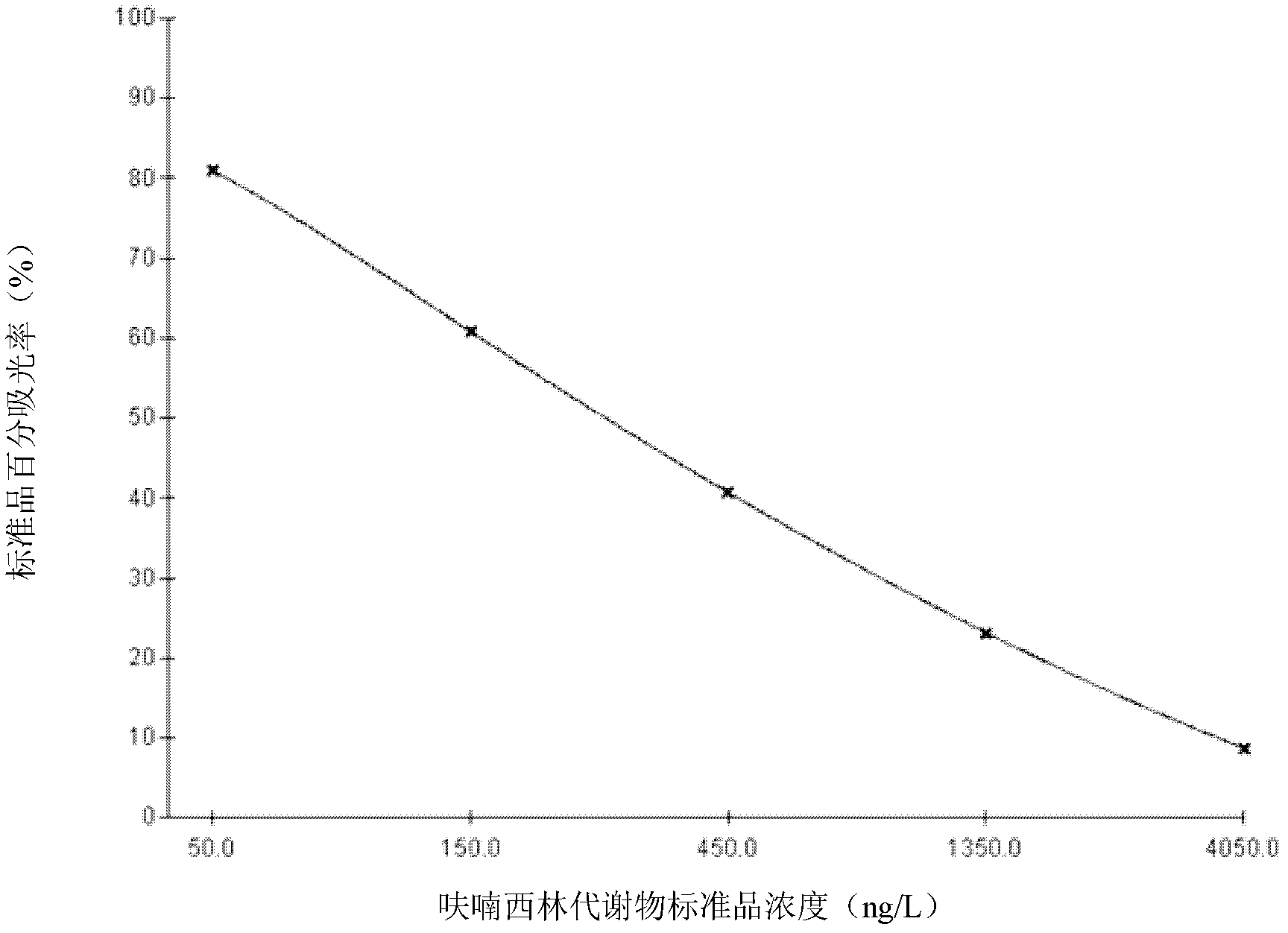
[0075] (4) Standard solution: The standard solution was prepared by gradient dilution method, and 6 bottles of series standard products were obtained, the concentrations were 0 μg / L, 0.05 μg / L, 0.15 μg / L, 0.45 μg / L, 1.35 μg / L, 4.05 μg / L, and high concentration standard 100μg / L, 1mL / bottle.
[0076] (5) The substrate chromogenic solution A is a carbamide peroxide solution, and the substrate chromogenic solution B is a tetramethylbenzidine solution.
[0077] (6) The stop solution is 2mol / L sulfuric acid solution.
[0078] (7) The concentrated washing solut...
Embodiment 3
[0081] Example three: detection of nitrofurazone metabolites in samples
[0082] 1. Pretreatment of samples
[0083] Pretreatment methods for tissue and aquatic samples
[0084] Weigh 1.0 g of the homogenized sample, add 4 ml of deionized water, 0.5 ml of 1M hydrochloric acid solution (weigh 8.3 ml of concentrated hydrochloric acid and add deionized water to make the volume to 100 ml) and 100 μl of derivatization reagent (injected with 2-nitro Add 10ml of methanol to the reagent bottle of benzaldehyde to dissolve and mix (concentration is 10mM)), shake fully with a shaker for 2min; incubate overnight at 37°C (about 16h); add 5ml of 0.1M dipotassium hydrogen phosphate solution (weigh 22.8 g dipotassium hydrogen phosphate trihydrate, add 1L deionized water to dissolve and mix), 0.4ml 1M sodium hydroxide solution (weigh 4.0g sodium hydroxide and 100ml deionized water to dissolve and mix), and 5ml ethyl acetate, shake Vigorously shake for 30s; over 3000g, centrifuge at room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com