Epoxide hydrolase mutant as well as gene and application of epoxide hydrolase mutant
An epoxide hydrolase and epoxide technology, applied in the field of bioengineering, can solve the problems of low catalytic activity and selectivity of naphthalene-based substrates, and achieve the effects of high enantioselectivity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
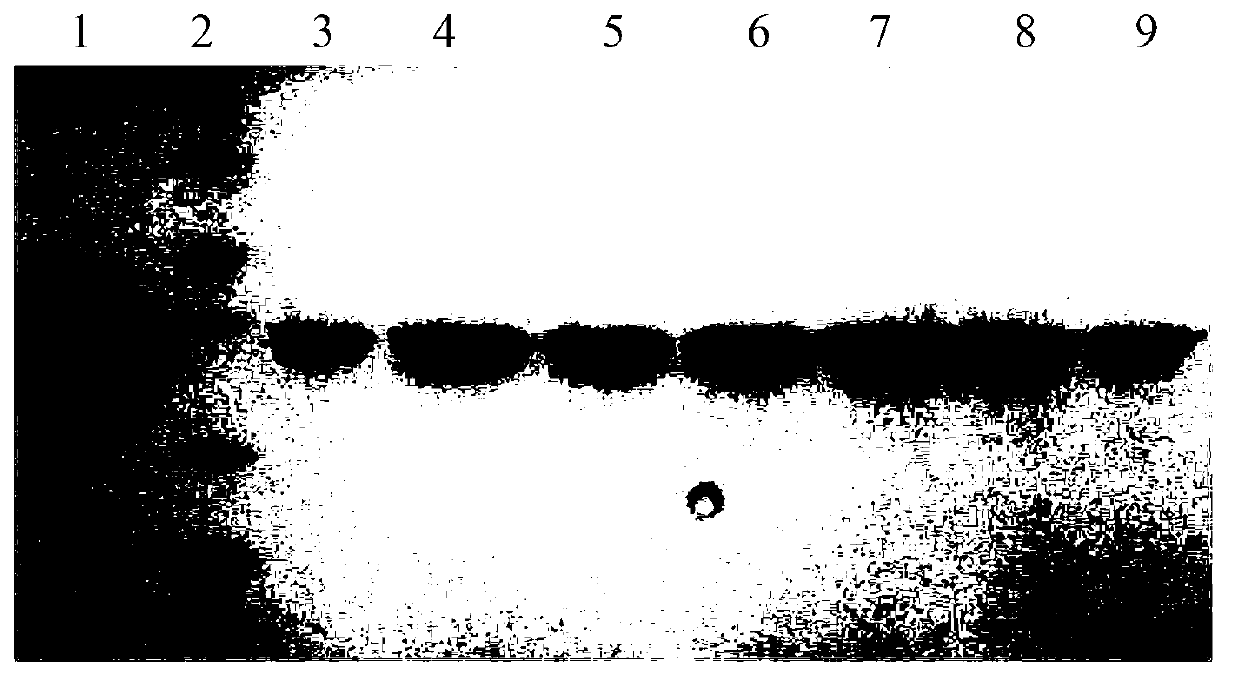
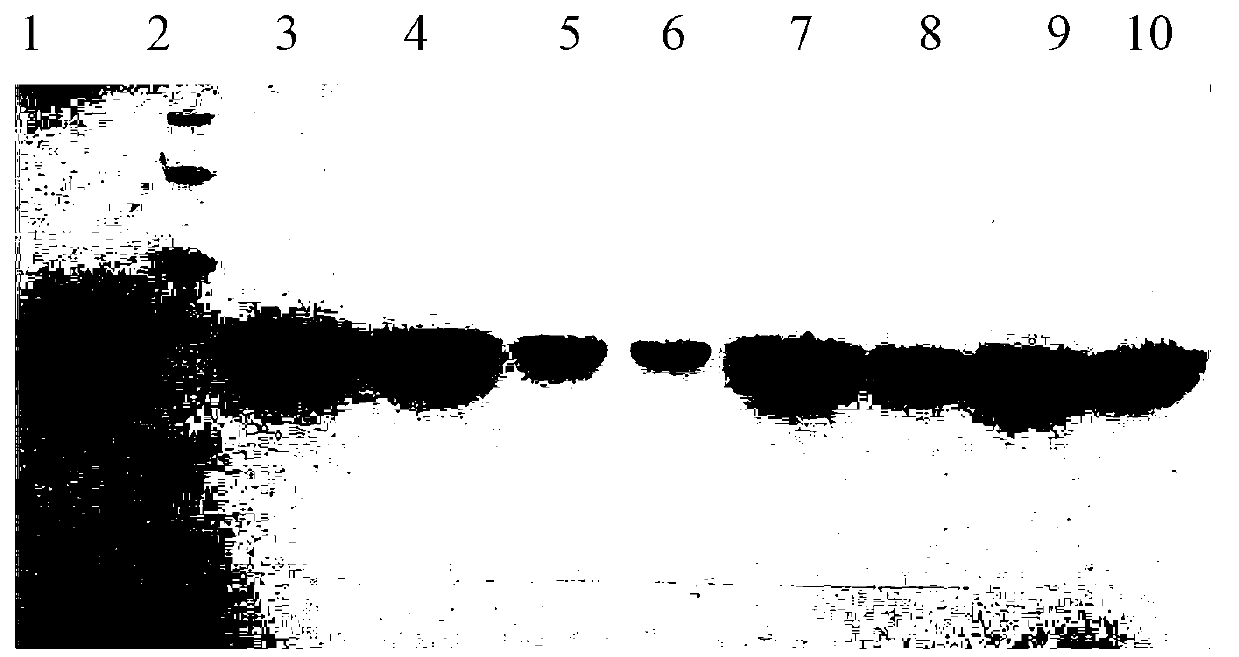
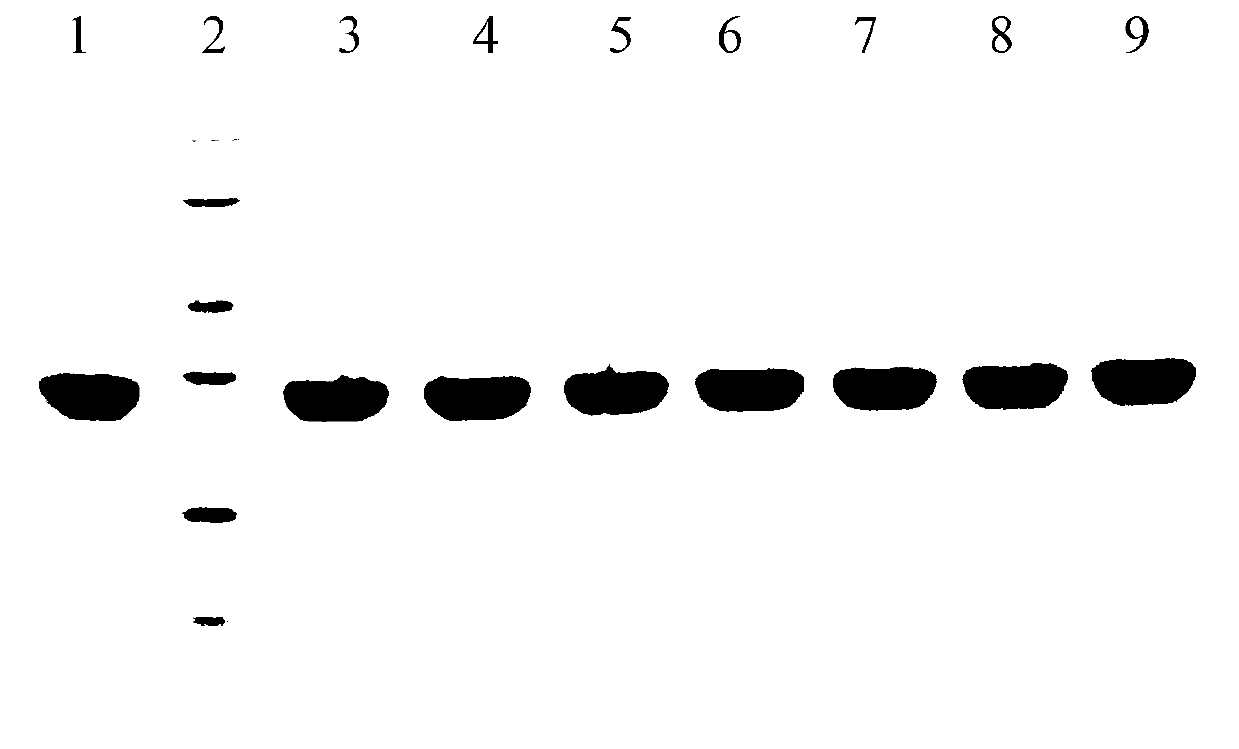
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 epoxyhydrolase mutant
[0046] Site-directed mutagenesis using II Site-Directed Mutagenesis Kit (Stratagene, Catalog #200522) described the protocol to operate. First design mutation primers containing mutation points, as shown in Table 1, including the 98th, 101st, 123rd, 128th, 132nd, 144th, 145th in the sequence shown in SEQ ID NO: 1 in the sequence table , 168, 169, 203, 206, 208, 219, 220, 221, 242 and 268 primers for introducing mutations, and saturation mutations at 128 and 145 (the 128th The phenylalanine is mutated to any of the 20 amino acids and the 145th methionine is mutated to any of the 20 amino acids and) two pairs of degenerate primers, the sequences of the above primers are shown in the sequence table Shown in SEQ ID NO:2~SEQ ID NO:43:
[0047] Table 1 Mutation primers of epoxyhydrolase
[0048]
[0049]
[0050] PCR reaction system (50μl): template 0.5~20ng, 5μl 10×KOD plus buffer, 5μl dNTP (each 2.0mM), 2μl ...
Embodiment 2
[0054] Example 2 Expression and Purification of Bacillus megaterium Epoxyhydrolase Mutant
[0055] 1. Expression of Epoxyhydrolase Mutants
[0056] The expression strain of the mutant obtained in Example 1 was inoculated in 1 L of LB liquid medium containing 50 μg / ml kanamycin, cultivated at 37° C. at 200 rpm until the OD600 reached 0.6 to 0.8, cooled to 16° C. and added with a final concentration of 0.4mM IPTG, continue to culture for 20h to induce expression. Collect bacteria by centrifugation at 6000×g for 10 minutes, resuspend in 50mM pH8.0 sodium phosphate buffer (containing 500mM NaCl and 5mM β-mercaptoethanol), homogenate under high pressure, and centrifuge at 30,000×g for 45 minutes, then take the supernatant to obtain Crude enzyme solution of epoxyhydrolase mutant protein. The obtained crude enzyme solution is freeze-dried to obtain the crude enzyme powder of the epoxyhydrolase mutant.
[0057] 2. Purification of Epoxyhydrolase Mutants
[0058] Use the Ni affinity...
Embodiment 3
[0065] The activity measurement of embodiment 3 epoxyhydrolase mutants
[0066] We use high performance liquid chromatography (HPLC) to separate the substrate and product in the reaction mixture, measure the decrease of substrate concentration and the increase of product concentration in the reaction system before and after the reaction, and determine the catalytic activity of epoxyhydrolase. One unit of enzyme activity is defined as the amount of enzyme required to hydrolyze 1 μmol of substrate per minute under the above reaction conditions. Specific activity is defined as the number of units of activity per milligram of protein.
[0067] The detection method of epoxyhydrolase activity is as follows: the total reaction system is 0.5ml, which contains 2mM substrate, co-solvent is 10% DMSO, 100mM phosphate buffer (pH7.0). In a 2ml EP tube, react at 30°C and 1000rpm. After preheating for 5 minutes, add enzyme solution that was also preheated at 30°C for 5 minutes. Take 100μl of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com