Method for preparing stable albumin nanoparticle
A technology of albumin nano and albumin, which is applied in the field of preparation of albumin nanoparticles, and can solve the problems of difficult delivery of pharmacologically active substances in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, preparation of disulfide bond-stabilized human serum albumin nanoparticles.
[0038] Using human serum albumin and reduced glutathione as raw materials, disulfide bond-stabilized human serum albumin nanoparticles were prepared.
[0039] The specific preparation method is as follows:
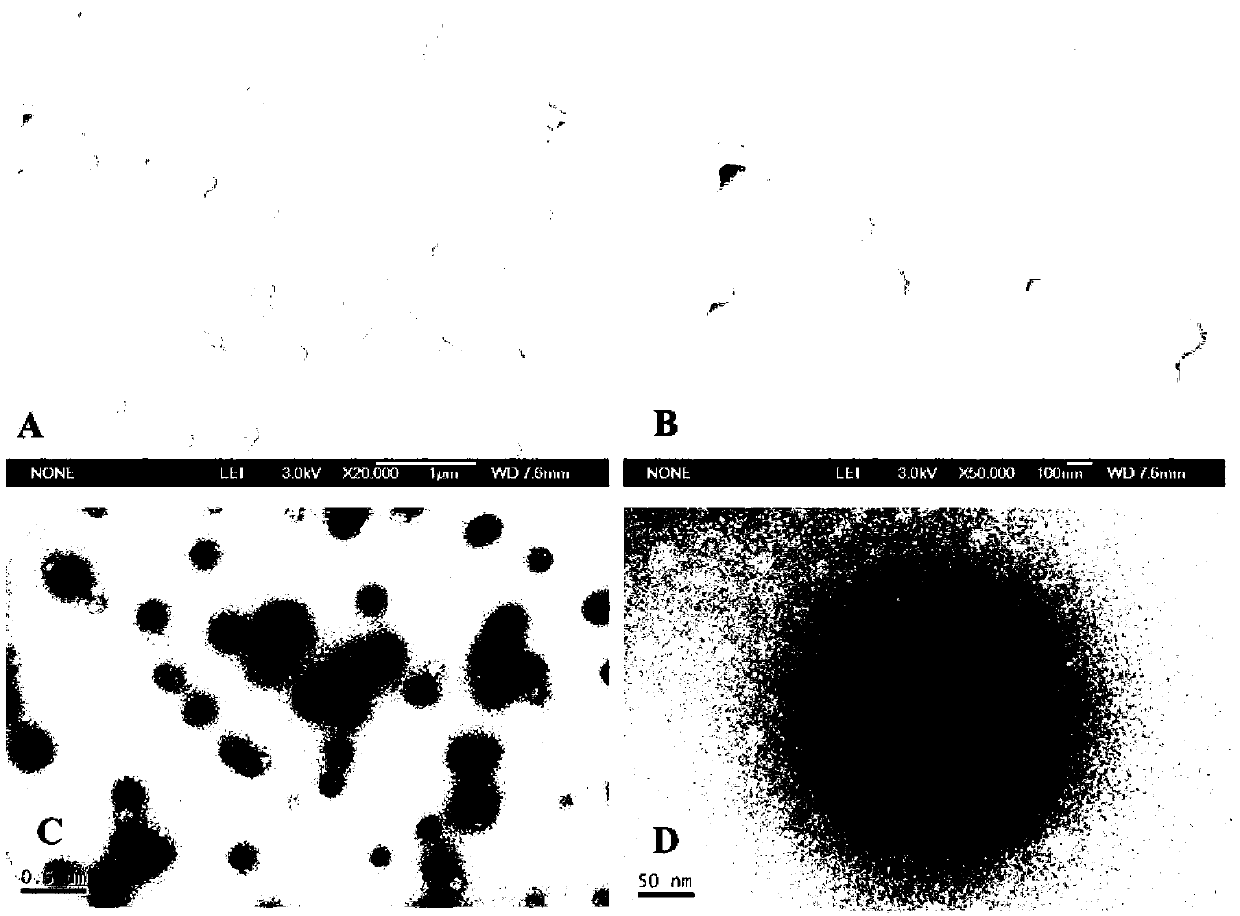
[0040]Dissolve 20 mg of human serum albumin in 1 mL of aqueous solution, add 20 mM reduced glutathione, react at room temperature for 1 hour, then add 4 mL of absolute ethanol to react for 30 minutes, and then dialyze the prepared suspension bag, dialyzed in deionized water at 4°C for 24 hours to obtain disulfide bond-stabilized human serum albumin nanoparticles, and the average particle size of albumin nanoparticles is 100-300nm (ZetaPALS, Zeta Potential Analyzer ).
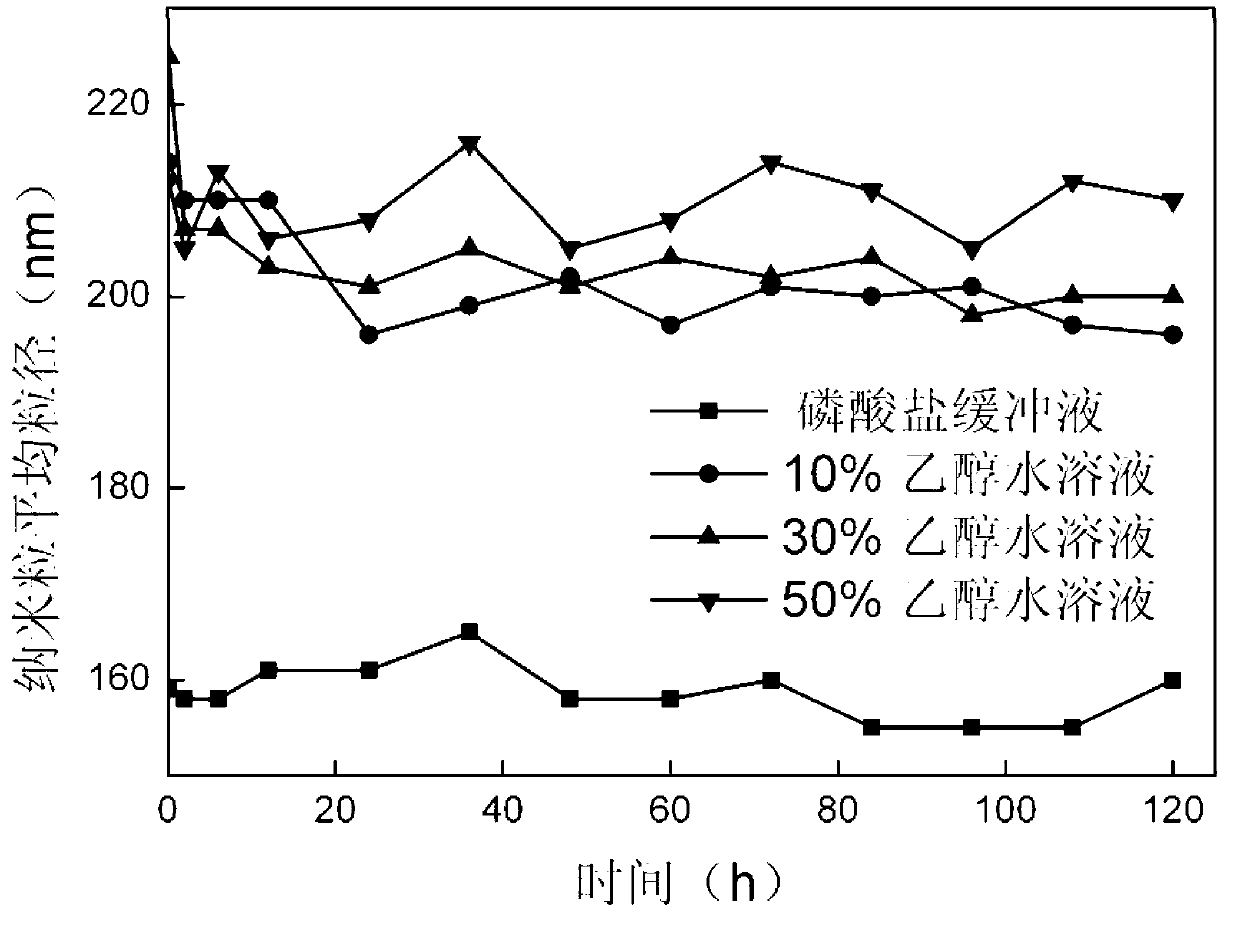
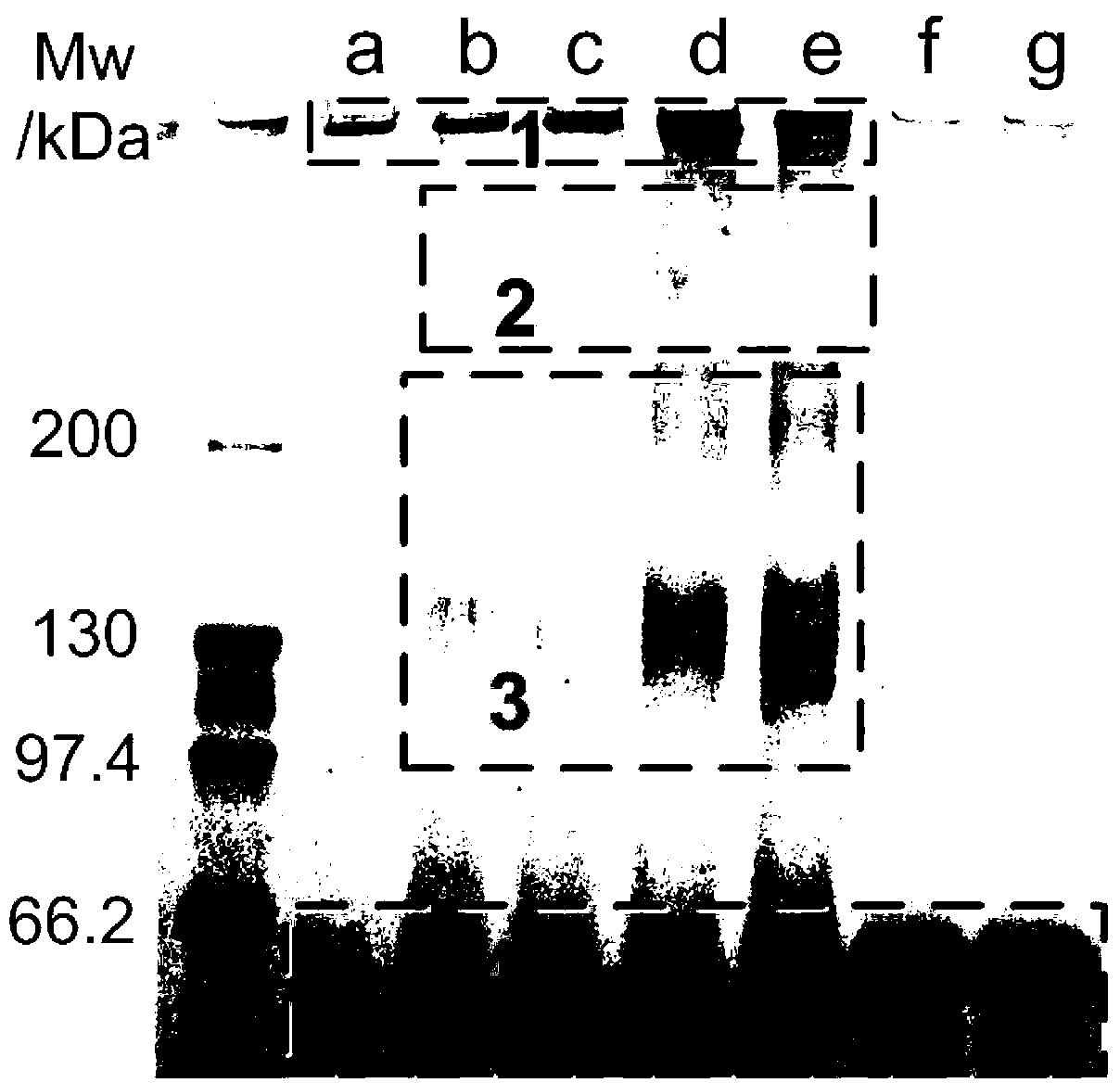
[0041] The stability of the prepared nanoparticles in different solvent environments can be obtained from figure 1 seen in. The stepwise dissolution process of the as-prepared nanoparticles under reducing envi...
Embodiment 2
[0042] Example 2, preparation of disulfide bond-stabilized human serum albumin nanoparticles.
[0043] Using human serum albumin and reduced glutathione as raw materials, disulfide bond-stabilized human serum albumin nanoparticles were prepared.
[0044] The specific preparation method is as follows:
[0045] Dissolve 200 mg of human serum albumin in 1 mL of aqueous solution, add 500 mM reduced glutathione, react at 50°C for 10 minutes, then add 1 mL of absolute ethanol for 5 minutes, and then dialyze the obtained suspension The bag is dialyzed in deionized water at 10° C. for 24 hours to obtain disulfide bond-stabilized human serum albumin nanoparticles, and the average particle size of the albumin nanoparticles is 100-400 nm.
Embodiment 3
[0046] Example 3, preparing a disulfide bond-stabilized curcumin-human serum albumin nanoparticle drug-loading system.
[0047] Human serum albumin, reduced glutathione and curcumin were used as raw materials to prepare a drug-carrying nanoparticle system of curcumin coated with human serum albumin stabilized by disulfide bonds.
[0048] The specific preparation method is as follows:
[0049] Dissolve 40 mg of human serum albumin in 1 mL of aqueous solution, add 50 mM reduced glutathione, react at room temperature for 1 hour, then add 4 mL of 3 mg / mL curcumin ethanol solution and react for 30 minutes, then prepare the obtained The suspension was placed in a dialysis bag, and dialyzed in deionized water at 4° C. for 24 hours to obtain disulfide bond-stabilized curcumin-human serum albumin-coated nanoparticles. Moreover, the average particle size of nanoparticles is 50-400nm (ZetaPALS, Zeta Potential Analyzer), and the drug-loading capacity of curcumin is determined by reversed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com