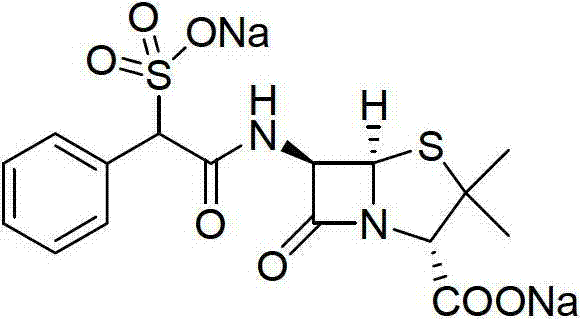
Method for preparing D (-)-sulbenicillin sodium
A technology of sulbenicillin sodium and sulbenicillin, which is applied in the direction of organic chemistry, can solve the problems of high production cost, poor solubility, and difficult industrialization, so as to avoid poor control of pH value, reduce the generation of impurities, and facilitate industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
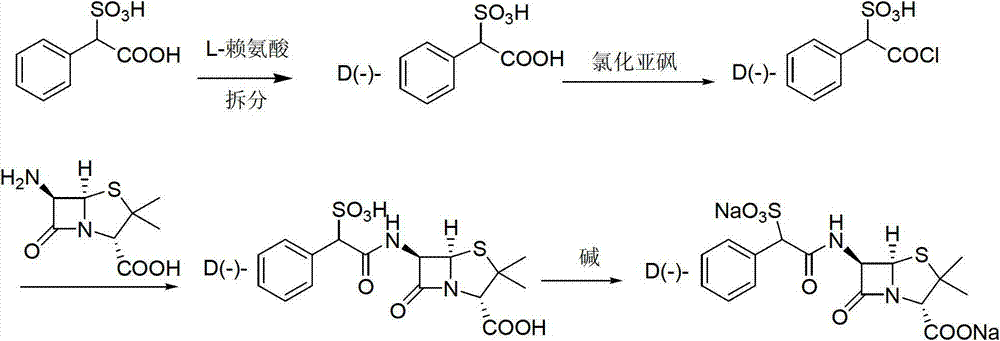
[0032] Embodiment 1: the preparation of compound a
[0033] Dissolve 100g (0.463mol) D(-)-sulfophenylacetic acid in 1000ml toluene, slowly add 150.9g chlorosulfonic acid (1.30mol) dropwise at room temperature, keep warm for 1.5 hours after the dropwise addition, and distill toluene under reduced pressure. Add 800ml of petroleum ether, stir for 0.5 hours, filter, and dry at 40-50°C for 4 hours to obtain 115.0g of compound a, with a yield of 98.1%, HPLC content of 98.6%, m.p: 65-68°C, ESI (m / z): 253.1.
Embodiment 2
[0034] Embodiment 2: the preparation of the compound b of acetyl protection
[0035] 100g of intermediate a (0.395mol) prepared in Example 1 was dissolved in 600ml of dichloromethane, and the dichloromethane solution of acetic anhydride was added dropwise at room temperature [dissolve 120.0g of acetic anhydride (1.175mol) in 100ml of dichloromethane in methane], react at room temperature for 1.0 hour after adding, after TLC detects that the reaction is complete, distill the solvent under reduced pressure, add 600ml of petroleum ether, stir for 0.5 hour, filter, and dry to obtain 115.4g of compound b protected by acetyl group, the yield 97.3%, HPLC content 98.1%, m.p: 87-90°C, ESI (m / z): 300.
Embodiment 3
[0036] Embodiment 3: the preparation of D (-)-sulfabenicillin sodium
[0037] Dissolve 100g of intermediate b (0.333mol) obtained in Example 2 in 600ml of dichloromethane, lower the temperature to 0~5°C, add 79.2g (0.366mol) of 6-APA in batches, and keep warm for 1.5 hours after the addition. After the TLC detection reaction was complete, dichloromethane was evaporated under reduced pressure, 300ml of water was added, 10% sulfuric acid was added dropwise under stirring to adjust the pH to 1.5~2.0, stirred for 1 hour, 400ml of n-butanol was added to extract once, and after standing for stratification Then add 400ml of n-butanol for extraction once, combine the organic phases, add 150ml of water to wash once, then add 200ml of water, control the temperature at 0~5°C, add dropwise 5% sodium bicarbonate solution to adjust the pH to 6.5-7.0, static After separation, the organic phase was washed with 50ml of water, the layers were allowed to stand, the aqueous phases were combined, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com