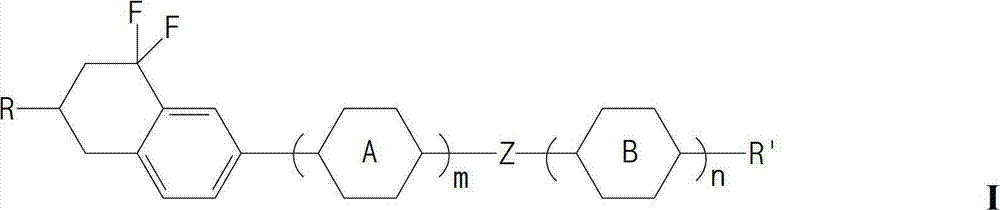
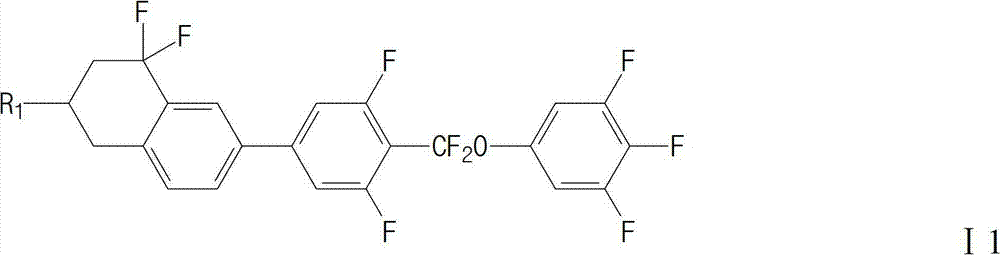
Liquid crystal compound and preparation method and application thereof
A liquid crystal compound and product technology, applied in the field of liquid crystal compound and its preparation, can solve the problems of insufficient charge retention rate, poor image quality, and insufficient fast response of TFT-LCD, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1 The synthetic method of 1,1-difluorotetrahydronaphthalene liquid crystal compound
[0078] 1.
[0079]
[0080] In a 500mL clean and dry three-neck flask, add 3.1g (0.13mol) magnesium chips, 10mLTHF and a small amount of bromopropane and stir to initiate the reaction, then add the remaining bromopropane (a total of 14.8g, 0.12mol) and 250mL THF dropwise at a temperature of about 25°C The formed solution was reacted at a temperature of about 25°C for 1.5 hours; the temperature was controlled at -5°C to 0°C, and a solution composed of 0.1mol p-bromophenylacetaldehyde and 50mL TFT was added dropwise, and the temperature was controlled for 1 hour to react. Slowly pour the reaction solution into a beaker filled with crushed ice and 50mL concentrated hydrochloric acid, acidify and hydrolyze, separate the liquid after stirring, extract the water phase with (50mL×2) toluene twice, combine the organic phase, and use (50mL×2) toluene for the organic phase 2) Wash...
Embodiment 2
[0100] 1. Synthesis
[0101] Using THF as a solvent, replacing bromopropane with bromoethane, the molar ratio of p-bromophenylacetaldehyde, bromoethane and magnesium chips is 1:1:1, and the temperature is controlled at -5°C to 0°C for 1 hour to obtain the compound Yield 88%.
[0102] 2. Synthesis
[0103] Under nitrogen protection, dichloromethane was used as solvent, pyridine, triphenylphosphine, React with bromine according to 1:1:1:1 at room temperature for 8 hours to obtain the compound The yield is 85%.
[0104] 3. Synthesis
[0105] With absolute ethanol as solvent, the compound Sodium metal and diethyl malonate 1:1:1 react at a temperature of 50°C-60°C for 1 hour to obtain the compound The yield is 83%.
[0106] 4. Synthesis
[0107] The compound obtained in step 3 and polyphosphoric acid were reacted for 2 hours at a ratio of 1:2.5 to obtain the compound The yield is 82%.
[0108] 5. Synthesis
[0109] Using dichloromethane as a solvent, the ...
Embodiment 3
[0116] 1. Synthesis
[0117] Use THF as a solvent, use bromopentane instead of bromopropane, the molar ratio of p-bromophenylacetaldehyde, bromoethane and magnesium chips is 1:2:2, and the temperature is controlled at -5°C to 0°C for 2 hours to obtain the compound The yield is 85%.
[0118] 2. Synthesis
[0119] Under the protection of nitrogen, dichloromethane is used as a solvent, pyridine, triphenylphosphine, the compound obtained in step (1) and bromine are treated according to the ratio of 1:5:5:5 at room temperature for 9 hours to obtain the compound The yield is 85%.
[0120] 3. Synthesis
[0121] Use absolute ethanol as a solvent, diethyl malonate, sodium metal and the compound obtained in step (2) in a molar ratio of 1:1.5:1.5 and react at a temperature of 50°C-60°C for 1.5 hours to obtain the compound The yield is 80%.
[0122] 4. Synthesis
[0123] The compound obtained in step 3 reacted with polyphosphoric acid 1:3 for 2.5 hours to obtain the compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com