Lacosamide tablet for treating epilepsy and preparation method for lacosamide tablet
A technology of lacosamide tablets and lacosamide, which is applied in the fields of pill delivery, sugar-coated pills, nervous system diseases, etc., can solve problems such as unstable properties and poor stability, and achieve good stability, stable source, and excipients safe and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
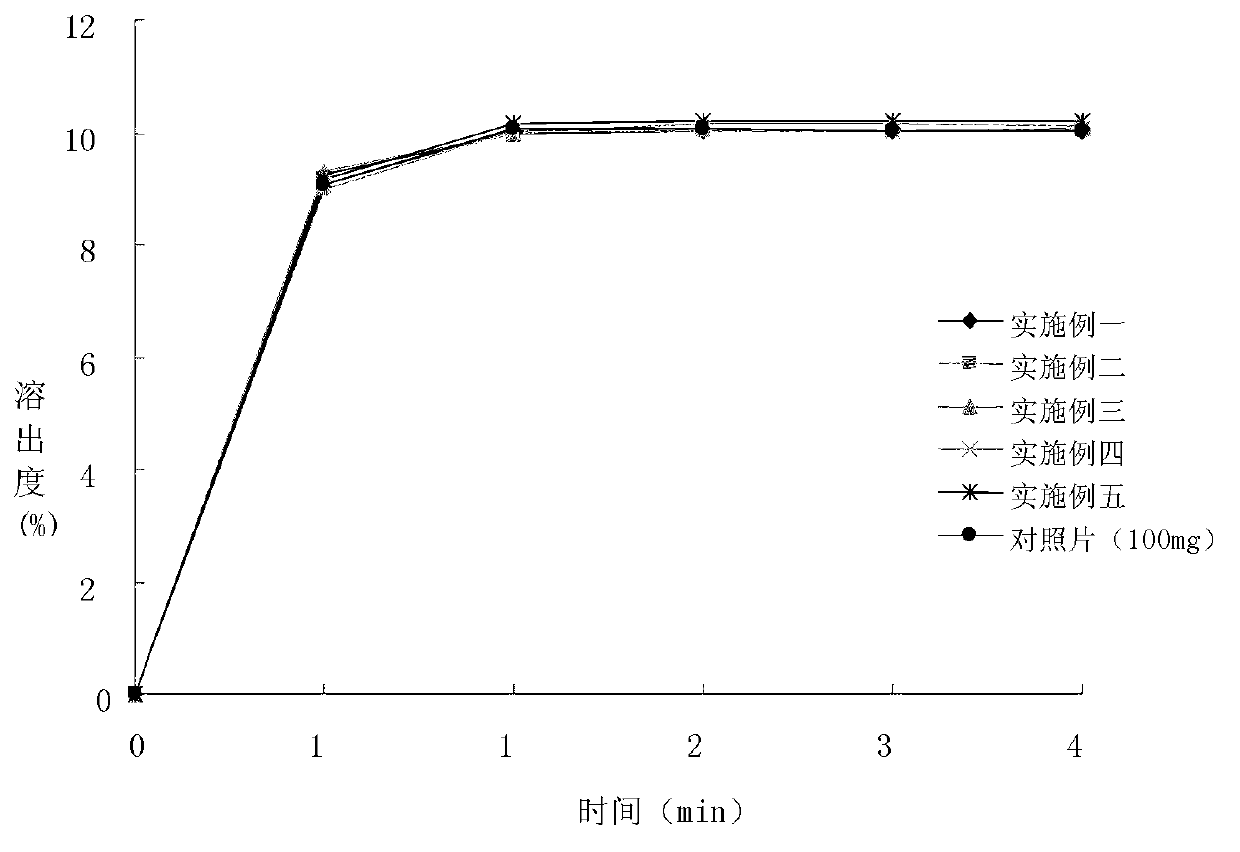
Image
Examples
Embodiment 1
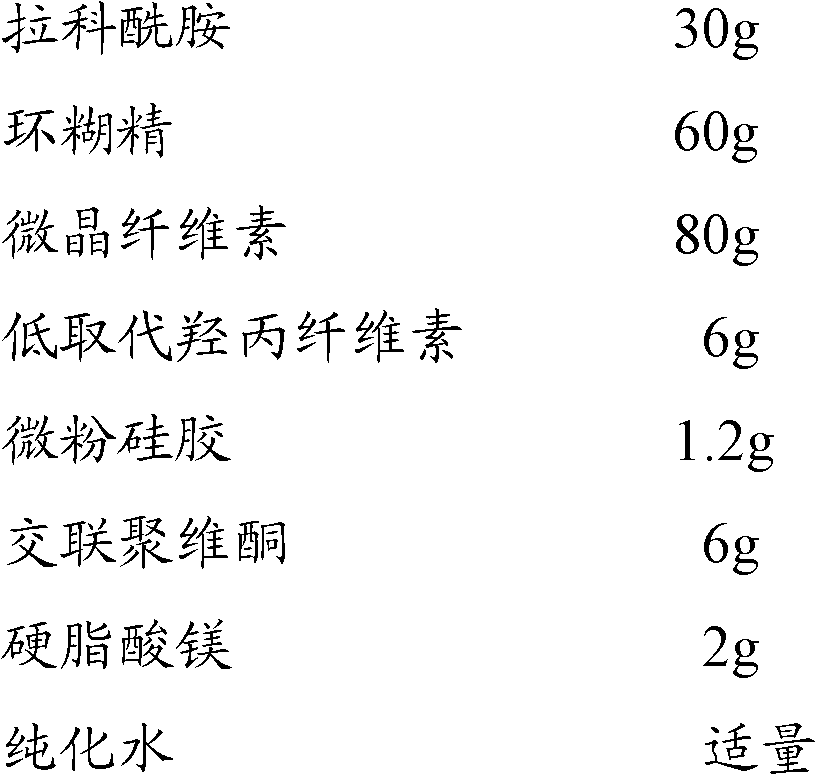
[0063] Prescription: (Specification 30mg, 1000 tablets)
[0064]
[0065]
[0066] Preparation Process:
[0067] 1. Dissolve 60g of cyclodextrin in 180ml of purified water, dissolve 30g of lacosamide in a small amount of purified water at 65-80°C, put the two solutions into a grinder and grind them into a paste, and dry them with air at 50-60°C to obtain Sample, mix the sample with 80g microcrystalline cellulose, 6g low-substituted hydroxypropyl cellulose, and 1.2g micropowder silica gel;
[0068] 2. Add an appropriate amount of purified water to the mixture obtained in 1 to prepare a soft material, pass through a 20-mesh sieve to granulate, dry, and granulate;
[0069] 3. Add 6g of crospovidone and 2g of magnesium stearate, mix well, and press into tablets;
[0070] 4. Coating, packaging, and full inspection.
Embodiment 2
[0072] Prescription: (Specification 50mg, 1000 tablets)
[0073]
[0074] Preparation Process:
[0075] 1. Dissolve 100g of cyclodextrin in 300ml of purified water, dissolve 50g of lacosamide in a small amount of purified water at 65-80°C, put the two solutions into a grinder and grind them into a paste, and dry them with air at 50-60°C to obtain Sample, this sample is mixed with 50g lactose, 50g starch, 5g micropowder silica gel;
[0076] 2. Add the mixture obtained in 1 into purified reclaimed water to prepare soft material, pass through a 20-mesh sieve to granulate, dry, and granulate;
[0077] 3. Add 10g of croscarmellose sodium and 3.0g of talc powder, mix well, and press into tablets;
[0078] 4. Coating, packaging, and full inspection.
Embodiment 3
[0080] Prescription: (Specification 70mg, 1000 tablets)
[0081]
[0082] Preparation Process:
[0083] 1. Dissolve 140g of cyclodextrin in 420ml of purified water, dissolve 70g of lacosamide in a small amount of purified water at 65-80°C, put the two solutions into a grinder and grind them into a paste, and dry them with air at 50-60°C to obtain Sample, mix the sample with 140g microcrystalline cellulose, 20g low-substituted hydroxypropyl cellulose, and 10g micropowder silica gel;
[0084] 2. Add the mixture obtained in 1 into purified reclaimed water to prepare soft material, pass through a 20-mesh sieve to granulate, dry, and granulate;
[0085] 3. Add 8g crospovidone and 2g magnesium stearate, mix evenly, and press into tablets;
[0086] 4. Coating, packaging, and full inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com