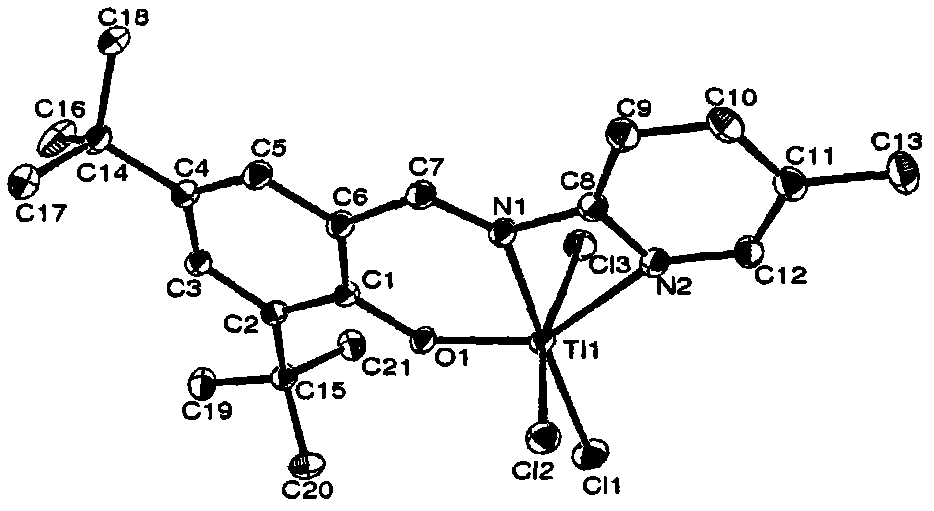
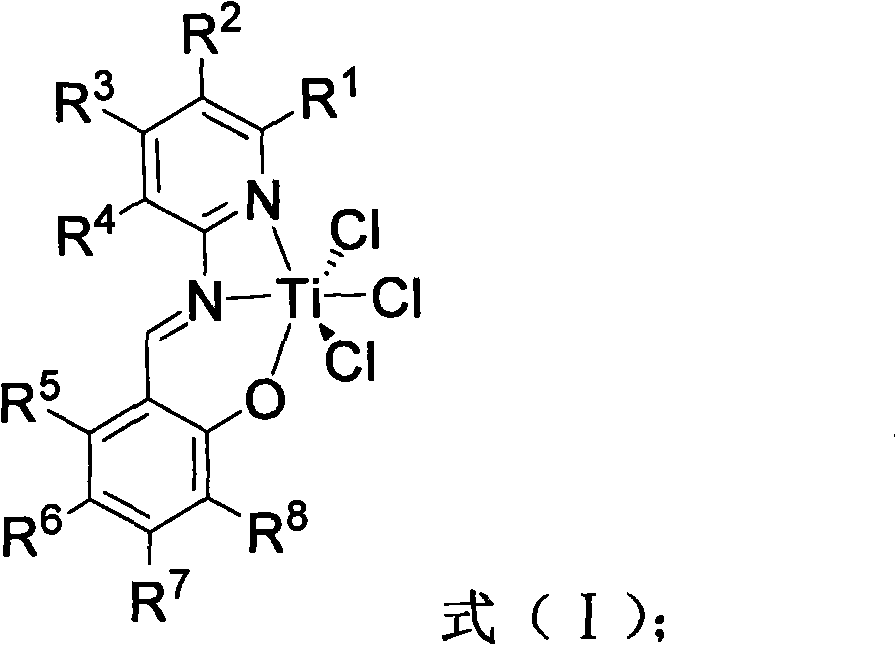
Salicylaldehyde pyridine imido titanium trichloride complex, its preparation method and application thereof, and polymerization method of ethene
A technology of salicylaldehyde pyridinimine titanium trichloride and pyridinimine, which is applied in the direction of titanium organic compounds to achieve the effect of high catalytic reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of salicylaldehyde pyridine imine titanium trichloride complex provided by the present invention comprises the following steps:
[0049] (1) Under the aldehyde-amine condensation reaction conditions, the salicylaldehyde of the structure shown in the formula (II) and the 2-aminopyridine type compound of the structure shown in the formula (III) are carried out the first contact reaction, obtain the formula (IV) The shown pyridine imine compound;
[0050]
[0051] Among them, R 1 -R 8 The definition of is the same as the above formula (I).
[0052] (2) Under the protection of an inert gas, the dry solution of the pyridine imine compound represented by the formula (IV) is subjected to a second contact reaction at a temperature of -25°C to 25°C with a metal hydride to obtain a suspension;
[0053] (3) At a temperature of -78°C to 25°C, the above suspension was mixed with TiCl 4 ·(THF) 2 After mixing, the third contact reaction is carried out un...
Embodiment 1
[0079] 1) 2.34g (10mmol) 3,5-di-tert-butyl salicylaldehyde and 0.94g (10mmol) 2-aminopyridine are mixed uniformly, placed in a microwave oven with a frequency of 800MHz, and placed on a medium-high fire (power of microwave irradiation) Heated at 200W) for 1 minute, took it out and cooled to room temperature, and heated at medium-high heat for 1 minute to obtain a red-black liquid. Recrystallized with ethanol to obtain 2.52 g of orange crystals of 3,5-di-tert-butyl salicylaldehyde 2-pyridinimine with a yield of 81.2%.
[0080] with IR, 1 H NMR, 13 C NMR, elemental analysis characterize the product structure of gained, and the results are as follows:
[0081] IR (KBr, cm -1 ): 3000(O-H)(w), 2960(s), 2906(m), 2867(m), 1610(CH=N)(m), 1579(s), 1461(s), 1432(s), 1360(m), 1198(m), 1169(s), 881(m), 791(m), 769(m), 736(m).
[0082] 1 H NMR: (CDCl 3 , 400MHz, ppm): δ13.90(s, 1H, OH), 9.47(s, 1H, CH=N), 8, 50(d, 1H, J=3.60Hz, Py-H), 7.76(t, 1H, J=7.56Hz, Py-H), 7.49(s, 1H, Ar-H),...
Embodiment 2
[0094] 1) Mix 2.34g (10mmol) 3,5-di-tert-butyl salicylaldehyde and 1.08g (10mmol) 2-amino-5-methylpyridine evenly, place it in a microwave oven with a frequency of 800MHz, and set it under medium-high heat After heating for 2 minutes, take it out and cool it to room temperature, and then heat it under medium-high heat for 2 minutes to obtain a red-black liquid. Recrystallized with ethanol to obtain 2.36 g of orange crystals of 3,5-di-tert-butyl salicylaldehyde-2-pyridine-5-methylimine with a yield of 73.8%.
[0095] with IR, 1 H NMR, 13 C NMR, elemental analysis characterize the product structure of gained, the result is as follows:
[0096] IR (KBr, cm -1 ): 2997(w), 2958(s), 2906(m), 2868(m), 1612(CH=N)(m), 1570(s), 1462(s), 1386(m), 1358(m ), 1251(m), 1169(s), 1022(m), 881(m), 828(m), 769(w), 680(w).
[0097] 1 H NMR: (CDCl 3 , 400MHz, ppm): δ13.93(s, 1H, OH), 9.42(s, 1H, CH=N), 8.31(s, 1H, Py-H), 7.56(d, 1H, J=7.88Hz, Py-H), 7.46(s, 1H, Ar-H), 7.34(s, 1H, Ar-H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com