Lipid ultrasound microbubble mediated adeno-associated virus gene transfection preparation and preparation technology thereof
An ultrasonic microbubble and virus technology, which is applied in the field of biomedicine to achieve the effect of increasing permeability and promoting gene transmembrane transfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
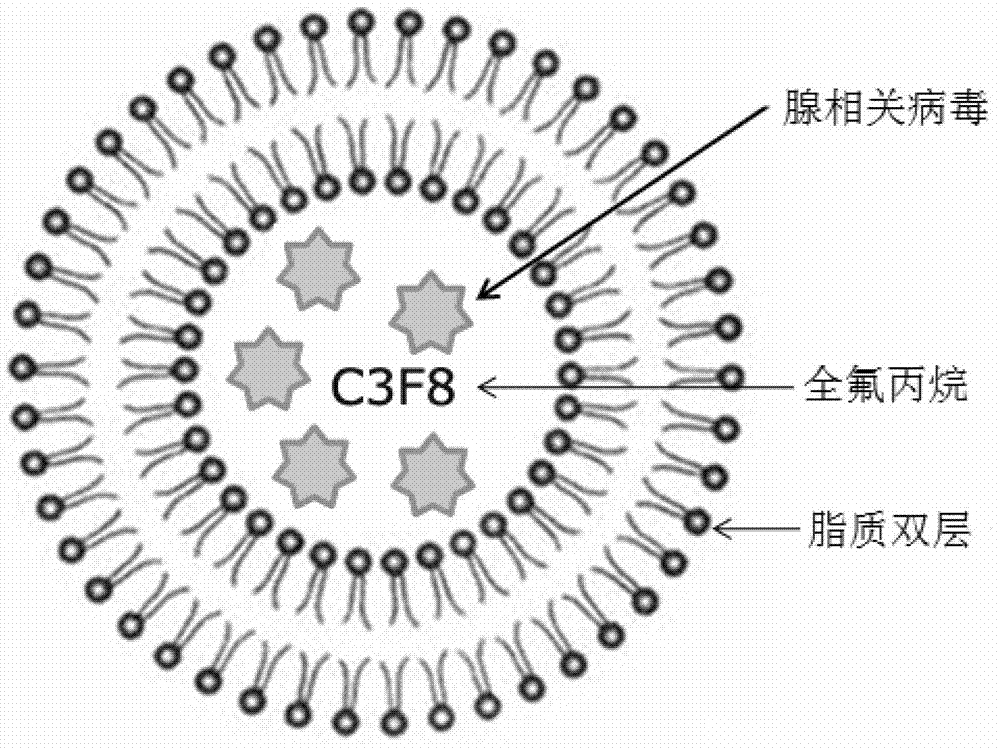
[0009] Precisely measure a certain proportion of AAV, phosphatidylcholine, phosphatidylethanolamine, cholesterol derivative cholesteryl hemisuccinate (CHEMS) and glycerol, dissolve in PBS, mix in water at 20°C for 10 min, and flush with perfluoropropane gas. Ultrasonication was performed with a 100W ultrasonic cleaner in a water bath at room temperature, with intervals of 10 s for 20 s, and a total of 10 cycles to prepare AAV-loaded lipid ultrasonic microbubbles.
[0010] Example 2: Morphological observation and particle size distribution detection of drug-loaded lipid microbubbles
Embodiment 2
[0011] The newly prepared lipid ultrasonic microbubbles were sealed and stored in a refrigerator at 4°C. They were taken out at 1h, 24h, 48h and 2 months respectively. After being properly diluted with PBS, they were placed under an inverted optical microscope to observe their appearance. To investigate the concentration of lipid ultrasonic microbubbles, take 1 drop of the diluent and place it on a hemocytometer, count the number of microbubbles in 8 large grids and take the average value (measured at least 3 times). And use the Malvern Sizer Nano ZS90 particle size analyzer to analyze the microbubble particle size and distribution according to the operating instructions. Observation with an inverted optical microscope showed that the lipid ultrasonic microbubbles prepared in this experiment were spherical and uniform in size. Place 1h, 24h and 48h after the lipid microbubble microscope observation result is consistent ( figure 2 ); Lipid microbubbles gathered into agglomera...
Embodiment 3
[0013] Human bronchial epithelial cells 16HBE14o-grow in DMEM / F12 (1:1) medium containing 10% fetal bovine serum, 100u / ml penicillin, 100μg / ml streptomycin, at 37°C, 5% CO 2 , cultured under saturated humidity conditions. The cryopreserved 16HBE14o- cells were cultured normally in a 150mm sterile cell culture dish after recovery.
[0014] Individually packaged Transwell chambers (clear polyester film, 0.4 μm) were placed in sterile 12-well cell culture plates. Take 16HBE14o-cells in a good growth state in the logarithmic growth phase, digest the monolayer culture cells with 0.25% trypsin for 2 min, and prepare a single cell suspension with DMEM / F12 (1:1) medium containing 10% fetal bovine serum. solution, 0.5ml per well, 3.5×10 6 Cells were inoculated in the inner chamber of Transwell, and 1.5 ml of complete medium was added to the outer chamber. Move the culture plate into CO 2 in an incubator at 37°C, 5% CO 2 Cultured under saturated humidity conditions to allow cells t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com