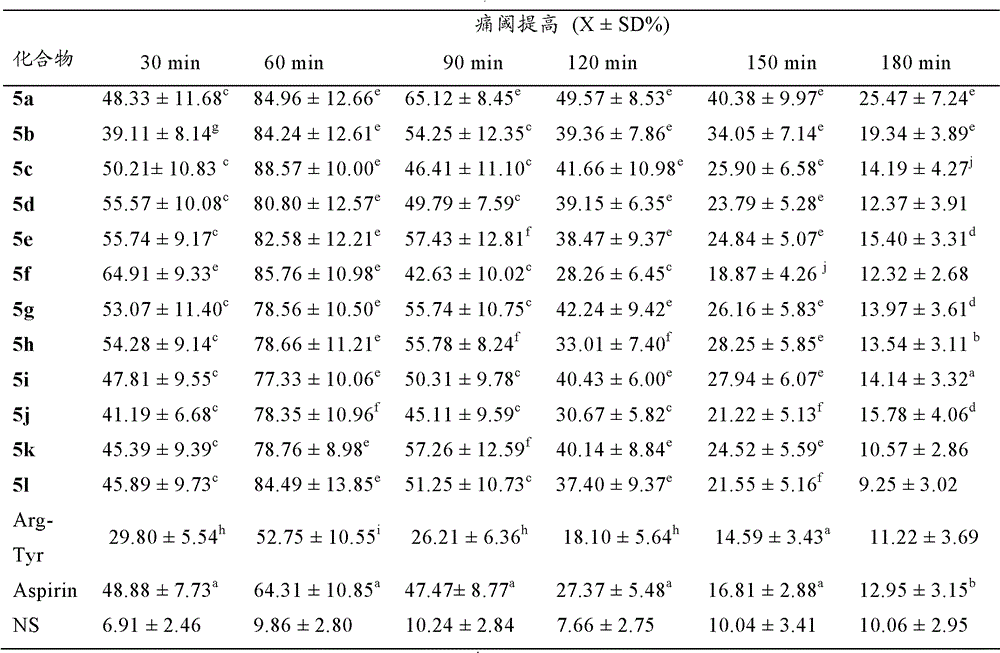
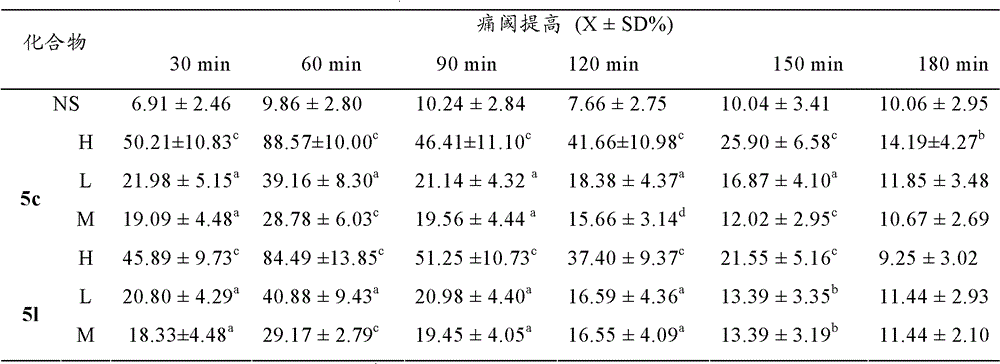
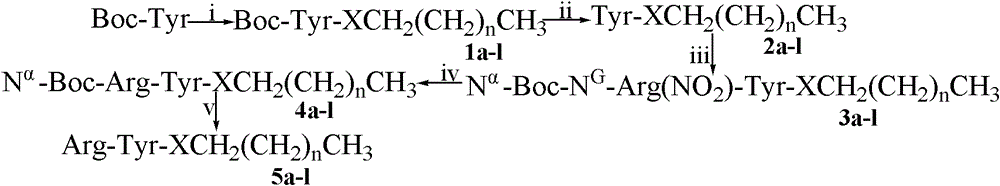Arg-Tyr modified aliphatic amine/ alcohol derivatives and preparation method and application thereof
A reaction and compound technology, applied in the field of artificially synthesized polypeptide compounds, can solve problems such as poor oral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 prepares N G -Arg(NO 2 )
[0022] Under the condition of ice-salt bath stirring, add 80ml concentrated nitric acid to 500ml eggplant bottle, then slowly add 80ml concentrated sulfuric acid. After 10 min, 47.33 g (272 mmol) of L-Arg was added in batches, and the reaction mixture was stirred and reacted for 2 h in an ice-salt bath. Slowly add 96ml of ice water to the reaction mixture, then slowly add concentrated ammonia water, adjust the pH to 8, then adjust the pH to 6 with glacial acetic acid, a large amount of colorless solid precipitates, put it in a refrigerator at 4°C to continue crystallization. Filter under reduced pressure, discard the filtrate, dissolve the filter cake with hot water at 80°C, and put it in a refrigerator at 4°C for recrystallization. Filter under reduced pressure at room temperature to collect 52.5 g of insoluble matter as a colorless solid with a yield of 88%. ESI-MS (m / e): 220 [M+H] + .
Embodiment 2
[0023] Embodiment 2 prepares N α -Boc-N G -Arg(NO 2 )
[0024] Weigh 1.752g N G -Arg(NO 2 ) into the reaction bottle, add 3ml of water to dissolve, under the condition of ice bath, slowly add 8ml of 1N NaOH dropwise, will dissolve 1.92g (Boc) 2 Slowly pour the dioxane of O into it, adjust the pH of the reaction solution to 9, and react for 48 hours. During this period, the CO in the reaction system is pumped every 2 hours 2 once. After the reaction is complete as detected by TLC, adjust the pH of the reaction solution to 7, spin off dioxane, then adjust the pH of the reaction solution to 2, extract the reaction solution three times with ethyl acetate, and then wash the ethyl acetate layer three times with saturated NaCl , to obtain the ethyl acetate layer with anhydrous NaSO 4 Dry overnight, filter to remove NaSO 4 , spin-dried ethyl acetate to obtain 2.4 g of the title compound as a colorless solid, yield 94%. ESI-MS (m / e): 320 [M+H] + .
Embodiment 3
[0025] Embodiment 3 prepares Tyr-OMe
[0026] Put 120ml of anhydrous methanol into the reaction flask, add 10.4ml of thionyl chloride dropwise under stirring in an ice bath, and dropwise add 7.24g (40mmol) of L-Tyr in batches within 30min, and stir the reaction mixture at room temperature for 24 hours, thin Layer chromatography was monitored until the disappearance of the starting material. The water pump decompressed and drained the reaction liquid, added methanol to dissolve, drained, and repeated 3 times; added ether, drained, and repeated 3 times. Recrystallized from methanol-ether, and filtered to obtain 8.78 g of the title compound as a colorless solid, with a yield of 87%. ESI-MS (m / e): 196 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com